Volume 20, Issue 1 (4-2023)
J Res Dev Nurs Midw 2023, 20(1): 66-72 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
chegini M, hajizade K, farshbaf A, lalooha F, Shahnazi M. Comparative efficacy of combined oral contraceptive capsules and vitamin D–combined oral contraceptive capsules on ovulatory dysfunction: A randomized clinical trial. J Res Dev Nurs Midw 2023; 20 (1) :66-72
URL: http://nmj.goums.ac.ir/article-1-1478-en.html
URL: http://nmj.goums.ac.ir/article-1-1478-en.html
1- Department of Midwifery, Faculty of Nursing and Midwifery, Qazvin University of Medical sciences, Qazvin, Iran
2- Department of Midwifery, Tabriz University of Medical Sciences, Tabriz, Iran
3- Physical Medicine and Rehabilitation Research Centre, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
4- Obstetrics and Gynecology Department, School of Medicine, Kosar Teaching Hospital, Qazvin University of Medical Sciences, Qazvin, Iran
5- Department of Midwifery, Faculty of Nursing and Midwifery, Tabriz University of Medical Sciences, Tabriz, Iran ,shahnazimahnaz@gmail.com
2- Department of Midwifery, Tabriz University of Medical Sciences, Tabriz, Iran
3- Physical Medicine and Rehabilitation Research Centre, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
4- Obstetrics and Gynecology Department, School of Medicine, Kosar Teaching Hospital, Qazvin University of Medical Sciences, Qazvin, Iran
5- Department of Midwifery, Faculty of Nursing and Midwifery, Tabriz University of Medical Sciences, Tabriz, Iran ,
Keywords: combined oral contraceptive, abnormal uterine bleeding (AUB), menstrual bleeding, vitamin D
Full-Text [PDF 481 kb]
(1220 Downloads)
| Abstract (HTML) (3270 Views)
Full-Text: (1480 Views)
Introduction
Abnormal uterine bleeding due to ovulatory dysfunction (AUB-O) is defined as excessive, long, and irregular bleeding of the endometrium without any pains or organ-related causes. It is often considered a sign of non-ovulation (1). Menstrual disorders account for 20% of all visits to the gynecologist's office (2). In fact, 35.8% of Iranian women have experienced one or more types of AUB (3). In Iran, a systematic meta-analysis reported the prevalence rates of polymenorrhea, hypermenorrhea, and menorrhagia at 9.94%, 12.94%, and 19.24%, respectively (4).
The complications of AUB-O can cause various psychosocial and physical outcomes, such as embarrassment, fear of unexpected bleeding, low quality of life, anger, unmotivated anxiety and aggression (5), fear of job loss and efficiency decline due to the frequent use of pads and tampons (6), high costs of surgical interventions and use of hygienic care resources (7), anemia of iron deficiency, fatigue, vertigo (8,9), endometrial hyperplasia, and carcinoma (10).
The primary objective of an AUB-O treatment is to stop excessive bleeding and prevent a relapse, whereas the secondary objective is to restart ovulation (10). The primary AUB-O management is the medical treatment, oral contraceptive pills (OCPs) are the preferred treatment for women of reproductive age (11). Other medical treatments include the use of antifibrinolytics, non-steroidal anti-inflammatory drugs (NSAIDs), gonadotropin-releasing hormone (GnRH) agonists, and surgical operation (8).
Despite their high costs and numerous side effects, none of these methods have definitively proven to be effective yet (12). Hence, it is essential to adopt an alternative treatment that could be safe, effective, available, and free of side effects (13).
Many believe in herbal medicines and food supplements, which can be used as natural treatments without any side effects or drug interactions. Many patients now seek alternatives to conventional treatments. Studies have shown that food intake and metabolism might play a key role in causing and treating menstrual disorders (14).
Vitamin D is a fat-soluble vitamin required for the appropriate functioning of the human body (15). The clinical diagnosis of vitamin D deficiency is established by measuring the 25(OH)D serum level (16). According to the definition of the World Health Organization (WHO) and the Institute of Medicine (IOM), a serum level of 25(OH)D of 30 ng/mL (75 nmol/L) or greater is considered sufficient, a level of 20-29 ng/mL (52-72.5 nmol/L) is considered insufficient, and a level of below 20 ng/mL (50 nmol/L) is considered deficient (17).
The biological actions of vitamin D are carried out through the vitamin D receptor (VDR), distributed in different tissues of the body (including the skeleton, parathyroid glands, and reproductive tissues). The vitamin D receptor is expressed in multiple reproductive tissues such as the placenta, uterine, and ovaries (17). According to recent data, vitamin D deficiency might be comorbid with several reproductive disorders, such as uterine fibroids, premature menarche (18), and polycystic ovary syndrome (PCOS) (17). In addition, women with PCOS have reported that vitamin D supplements make menstruation normal and improve the folliculogenesis of ovaries and ovulation (19). According to other studies, dietary vitamin D deficiency can weaken fertility in 2 species of mice (17). In fact, the mice that lack the necessary enzyme to convert the rotational form of vitamin D into its active form have shown signs of estrous cycle disorders, such as stalled follicular growth, prolonged estrous cycle (the period of preparedness for reproduction in female mammals), and lack of ovulation (20).
The relationship between vitamin D and the menstrual cycle length might be due to the anti-Mullerian hormone (AMH), which helps regulate the use of ovarian follicles (21). In fact, AMH is generated in the granulosa cells of growing follicles to hypothetically inhibit the absorption of initial follicles and decelerate the growth of follicles, thereby delaying or preventing atresia and inhibiting the differentiation of granulosa cells (22). Studies have also indicated that vitamin D can regulate AMH signaling in human luteinized granulosa cells. In other words, vitamin D supplements reduce the unnatural AMH serum levels in women with PCOS and vitamin deficiency, indicating the folliculogenesis improvement mechanism of vitamin D (23).
Vitamin D deficiency is among the most prevalent health problems worldwide (24). In fact, the risk of vitamin D deficiency (<30 ng/mL) threatens 69.4% of women in the reproductive age range (25). According to a review study in Iran, vitamin D deficiency rates were reported at 61.90% and 60.45% in non-pregnant and pregnant women, respectively (26). Another review study concluded that women had lower serum levels of this vitamin than men due to their dressing habits (27).
The results of a cross-sectional study indicated that a decreased concentration of 25(OH)D in plasma was associated with an increased chance of an irregular menstruation cycle (28). According to a preliminary study, lower vitamin D levels were correlated with 40% of long menstrual cycles, 27% of oligomenorrhea, and 13% of amenorrhea (29). Another descriptive study reported that increasing the levels of vitamin D would decrease the chances of long menstrual cycles (30). Moreover, a review study reported a strong correlation between vitamin D deficiency and a wide spectrum of polymorphic clinical manifestations, such as menstrual dysfunctions (31).
Having adverse effects on the quality of life, menstrual disorders are highly prevalent among women of productive age. According to the results of reviewing reputable databases of medical references, no study has yet analyzed the effects of combined oral contraceptives (COCs) with and without vitamin D on AUB-O. Given the limited portions of vitamin D in food products, low costs of consuming vitamins, and popularity of vitamins among patients, we decided to study the therapeutic effects of COC capsules with and without vitamin D on menstrual disorders among women of reproductive age.
Methods
The screened people included 102 women of reproductive age, 42 of whom did not meet the inclusion criteria (Flowchart 1).
.PNG)
Study design and sampling
This triple-blind clinical trial was conducted on 60 women of reproductive age (18-45 years old) from June 2021 to February 2022. This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (code: IR.TBZMED.REC.1399.1041), and it was registered at the Iranian Registry of Clinical Trials (IRCT20110606006709N22). Then, sampling permits were granted by the Research Department of Nursing-Midwifery School of Tabriz University of Medical Sciences and Kowsar Hospital in Qazvin.
The convenience sampling method was used to recruit eligible women. Women attending the gynecology clinic were approached by the researcher and given information about the study. Women who expressed interest in participating were asked to complete a checklist of inclusion and exclusion criteria. The target population included the women referring to the gynecology clinic of Kowsar Hospital in Qazvin, Iran. Inclusion criteria were women of reproductive age (18-45 years old) who had been experiencing at least 2 to 3 months of excessive, long, and irregular bleeding (without regular menstrual cycles [28-35 days]) and heaving bleeding for more than 7 days (11). Exclusion criteria were pregnancy, history of any cervix abnormalities (eg, cervical cancer, infection, trauma, polyp, and so on), daily and regular consumption of vitamin D supplements over the past 3 months, consumption of drugs affecting menstrual cycle and bleeding (eg, oral hormonal contraceptives, anticoagulants, selective serotonin reuptake inhibitors, antipsychotics, corticosteroids, hormonal supplements, phenytoin, soy herbal supplements, and ginseng), and any absolute or relative contradictions with combined contraceptive pills, pathological symptoms of the pelvis, pharmaceutical causes, systemic diseases, self-reported unnatural menstruation cycles (ie, irregular cycles followed by prolonged heavy bleeding with a history of such complaints for at least 2 to 3 cycles), and a specialist’s diagnosis at the gynecological clinic.
First, the pregnancy was ruled out by a β-HCG test. Pelvic pathological symptoms were excluded through ultrasonography, endometrial biopsy, Pap test, and pelvic examinations. Patients with a history of thyroid dysfunctions, coagulation disorders, systemic diseases, malignant conditions, and consumption of drugs affecting menstrual cycle and bleeding (eg, oral hormonal contraceptive) over the past 3 months were excluded.
After analyzing the inclusion and exclusion checklists, eligible women were selected and provided with information about the benefits and risks of the study. Participation was voluntary, and the women were asked to sign written consent forms before participating in the study. At the start of the study, each participant was asked to complete a researcher-made questionnaire to obtain accurate information about the menstrual cycle, including cycle length (normal: 21-35 days and abnormal: less than 21 or more than 35 days), bleeding duration (normal: 7 days or less and abnormal: more than 7 days), bleeding volume (using the Higham table), and history of clot expulsion.
All patients were instructed to keep a menstruation calendar. If participants were taking medications that could affect their menstrual cycle or bleeding, they were instructed to provide this information on the questionnaire. Participants were also asked to complete and return a questionnaire gathering personal and demographic information. They were then given the Menstrual Symptoms Questionnaire (MSQ), Higham Questionnaire (to assess bleeding intensity), and the Semi-Quantitative Vitamin D Food Frequency Questionnaire to complete at home for 1 menstrual cycle prior to beginning the intervention.
Tools
1. Demographic Questionnaire: This questionnaire includes different items about age, education, social status, economic status, and some other pieces of information. The participants completed the questionnaire after they entered the research process.
2. Menstrual Symptoms Questionnaire: This questionnaire includes the bleeding period and the menstrual cycle length. It was completed by participants before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle).
3. Higham Questionnaire: The Higham visual chart or the Pictorial Blood Loss Assessment Chart (PBAC) was employed to measure menstrual bleeding. It is an accurate but simple tool for assessing menstrual blood loss through a pictorial chart in which the number of pads and tampons used is recorded by measuring how much blood they are stained with (32). This tool is scored on a specific system, in which a score of 1 is given to a menstrual pad stained with a little blood, a score of 5 is given to a menstrual pad stained with an average amount of blood, and a score of 20 is given to a menstrual pad stained completely with blood. Every small stain (smaller than a circle with a diameter of 2 cm) is scored 1, whereas every wide stain (wider than a circle with a diameter of 3.5 cm) is scored 5. Furthermore, a blood spillage is scored 5. This questionnaire was completed by participants before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle). This chart is a non-laboratory simple method for the diagnosis of menorrhagia. It is a general scoring system that involves observing pads, tampons, and clots appearing as the basis. It is the best tool available for assessing menstrual blood loss, with 86% sensitivity and 89% accuracy (32). Since the Higham chart is valid, there was no need for its retest validity. To determine the reliability, a test-retest was used with 10 days intervals on 20 subjects (r = 0.85).
4. Semi-Quantitative Vitamin D Food Frequency Questionnaire: This questionnaire is completed and analyzed based on the intake of various food portions and their consumption frequency on a daily, weekly, monthly, yearly, “seldom,” or “never” basis. It was used in this study to evaluate the consumption of foods containing vitamin D (33) to make the necessary adjustments between groups in case of differences. For analysis, the daily food intakes (after converting the recorded food into grams) were measured and entered into Nutritionist IV software. The study groups were compared in terms of the mean of those values. To assess reliability, the intraclass correlation coefficient (95% CI) was calculated using the 2-week test-retest method on a sample of 20 subjects, resulting in a coefficient of 0.88 (0.88-0.93).
5. Moreover, the medicine side effect checklist was completed by participants during the intervention (3 cycles), and the satisfaction checklist during 1 cycle of medicine. The medicine side effect checklist included headache, dizziness, visual and nervous disorders, nausea, vomiting, thirst and frequent urination, fatigue, dry mouth, mood changes, breast tenderness, spotting, weight gain, digestive system disorders, and other symptoms.
6. The Checklist of Satisfaction with the Received Medicine: This questionnaire was completed by the participants after the end of the intervention. It included 5 questions ranging from completely satisfied to very dissatisfied.
The content and face validities were then used to determine validity. For this purpose, the questionnaires were given to designated faculty members, and their feedback was collected to make any necessary modifications to the tools. The research tools were standard and have been shown to be reliable in previous relevant studies. Regarding the Higham Questionnaire, Zakherah et al (2011) indicated significant correlations (α = 0.6) between the PBAC scores and the menstrual blood loss measured by the hematinic phosphatase test (used as a gold standard to compare the blood loss from menstrual hygiene products with the venous blood sample) (34).
A randomized block sampling method in blocks of 4 or 6 members was used to assign eligible participants to the control group (ie, the LD group) and intervention group (ie, the LD + vitamin D group) with a 1:1 allocation ratio. An expert who was not part of the research team used Random Allocation Software (RAS) to determine the random allocation sequence, and medication packets numbered 1 to 60 were provided in accordance with this sequence. The researcher, patients, data analyst, and outcome analyst knew nothing about the contents of the packets. The packets that contained the capsules were uniform, sealed, and blurred. Based on the allocation sequence, they were prepared by an individual who was not part of the research team.
The control group received 21 LD capsules (containing 30 µg of ethinylestradiol and 150 µg of levonorgestrel available in Iranian drugstores under serial number 148 by Iran Hormone Pharmaceutical Laboratory) and 9 placebo capsules (containing starch and lactose) in each cycle. The intervention group received 21 combinatory capsules containing vitamin D (1000 units of cholecalciferol; Alborz Daru Company, Qazvin, Iran) and LD (containing 30 µg of ethinylestradiol and 150 µg of levonorgestrel), as well as 9 vitamin D capsules (1000 units of cholecalciferol) to be taken once per day. They were placed separately in similar packets for consumption in 3 cycles. The appearances of vitamin D3, LD, and placebo capsules were altered by dividing the abovementioned drugs into capsule coverings under the supervision of a pharmacist at Alborz Daru Company. In fact, they were given in exactly uniform shapes to the women in the intervention and control groups. To maintain acceptance, the participants were given the drugs for only 1 month and were then informed that they would be given the rest of the drugs again in the next follow-up periods. They were provided with the packets and daily consumption checklist the research questionnaires. They were also told to keep taking 1 capsule per day. The patients were followed up for 3 consecutive cycles during the intervention period and for 1 cycle after completion of the intervention. In addition to the menstruation pattern assessed in the final cycle, drug satisfaction was analyzed using the questionnaires.
One month after the end of the intervention, the researcher collected the questionnaires completed in the final cycle in accordance with the arrangements made via phone calls. Moreover, the participants were followed up every 2 weeks during the intervention period through phone calls.
Sample size
According to Mehrabian et al (35) and based on the mean difference of the 2 independent groups, a 2-tailed test was conducted in G*Power 3.1.2 to assign 25 participants to each group for the menstrual blood loss variable by considering m1 = 18.8, m2 = 15.04, sd1 = 15.04, and sd2 = 4.6 with the power of 80% and α = 0.05. Regarding the number of menstrual days, a 2-tailed test was conducted to assign 17 participants to each group by considering m1 = 9.6, m2 = 7.68, sd1 = 15.04, and sd2 = 1.9 with the power of 80% and α = 0.05. The final sample size was considered 30 participants in each group with respect to a potential attrition rate of 20%.
Statistical methods
After data collection, descriptive statistics, such as frequency (percentage), mean (±SD), and median (25-75 percentile), in addition to analytical statistics (eg, Mann-Whitney U test, Friedman test, Fisher exact test, independent t test, and Cochran Q test) were used in SPSS version 24 (SPSS Inc, Chicago, IL, USA) for data analysis. The normality of the data distribution was checked using the Kolmogorov-Smirnov test. P values less than 0.05 were considered statistically significant.
Results
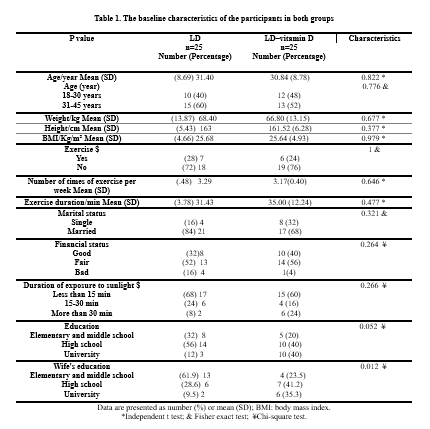
The baseline characteristics of the participants are shown in Table 1. According to the statistical tests, there were no significant differences between the 2 groups in terms of demographic information and social characteristics of women (P > 0.05; Table 1).
.PNG)
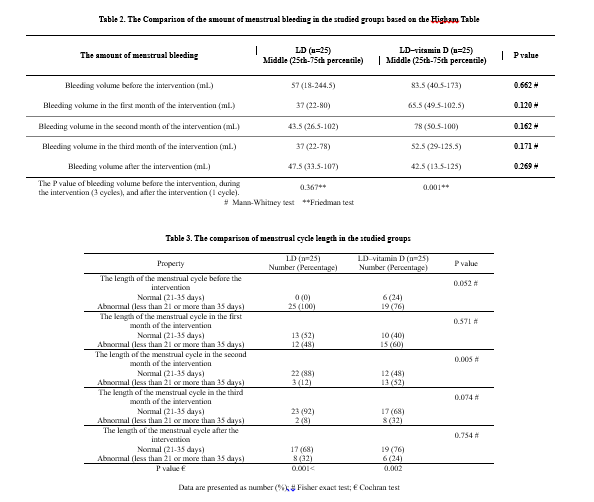
The medians (25-75 percentile) of pre-intervention blood loss were 57 (18-244.5) and 83.5 (40.5-173) in the control and intervention groups, respectively. The post-intervention medians were reported to be 47.5 (33.5-107) and 42.5 (13.5-125) in the control and intervention groups, respectively. According to the Friedman test results, there were no significant differences in the control group before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; P = 0.367) in terms of blood loss. However, there were significant differences in the intervention group in terms of blood loss before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; P = 0.001; Figure 1 and Table 2).
According to the Cochran Q test, there were significant improvements in the control group (P < 0.001) and intervention group (P = 0.002) in terms of the menstrual cycle length during the trial (before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; Table 3).
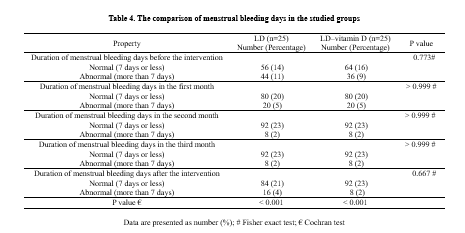
According to the Cochran Q test, there were significant improvements within the control and intervention groups in the number of menstrual bleeding days during the study (before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; P < 0.001; Table 4).
The means (±SDs) of vitamin D intakes were 1.368 (±0.628) mg/day and 1.426 (±0.375) mg/day in the control and intervention groups, respectively. The mean difference was 0.058 (95% CI, -0.32 to 0.44). According to the independent t test, there were no significant differences between the study groups in terms of vitamin D intake through diets (P = 0.763).
Less than one-fourth of the control group (16%) and half of the intervention group (52%) were completely satisfied with the consumption of the prescribed drugs, whereas most of the control cases (80%) and nearly half of the intervention cases (48%) were satisfied with the treatment. However, none of the women in the intervention group was satisfied with the treatment. According to the Mann-Whitney U test, there were significant differences between the 2 groups (P = 0.006).
The research results indicated no significant differences between the 2 groups in the frequency of medicinal side effects (eg, headaches [64% vs 60%], vertigo [60% vs 52%], visual and neurological disorders [52% vs 48%], fatigue [84% vs 60%], nausea and vomiting [24% vs 44%], thirst and frequent urination [52% vs 40%], xerostomia [52% vs 60%], mood changes [80% vs 76%], breast sensitivity [36% vs 48%], spotting [44% vs 56%], weight gain [40% vs 44%], gastrointestinal disorders [44% vs 44%], and backaches [4% vs 2%]) in the intervention and control groups, respectively.
Discussion
This study aimed to compare the effect of COC capsules and vitamin D–COC capsules on ovulatory dysfunction in reproductive-age women with AUB-O.
According to the results, menstrual blood loss decreased in the intervention group than in the control group; however, the difference was not significant. Over time, both intervention and control groups showed a significant improvement in bleeding duration and menstrual cycle length, resulting in a more normal menstrual cycle. However, the intervention group showed more normal bleeding duration and menstrual cycle length for a longer period compared to the control group.
The menstrual blood loss comparison results indicated that in both groups, participants experienced less blood loss in each cycle compared to the previous cycle.
In a randomized controlled trial, Adawiyah et al (2020) compared the effect of tranexamic acid and COC on 60 women using depot medroxyprogesterone acetate (DMPA) with AUB, showing that the average length of bleeding was 3.62 ± 5.2 days in the tranexamic acid group and 6.76 ± 9.2 days in the COC group, which was statistically significant (P = 0.018) (36).
In a randomized controlled trial, Sandhya Jain et al (2016) compared the effect of the intravaginal ring and COC on 60 women for 3 consecutive months, showing that blood volume was significantly reduced in both groups; however, although the reduction in Higham scores was higher in the group that received the intravaginal ring, this difference was not statistically significant (P = 0.0286) (37).
Rahmanian et al (2018) conducted a controlled randomized trial on the effect of vitamin D on uterine leiomyoma among 51 women, indicating a significant reduction in the menstrual blood loss of the intervention group (receiving vitamin D) than in the control group (receiving placebo); however, there were no significant differences in the menstrual blood loss between the 2 groups. This finding is consistent with our results (38).
Zarei et al (2017) conducted a controlled randomized clinical study on the effects of calcium + vitamin D and calcium alone on menstrual bleeding among 85 girls aged 18-32 years with primary dysmenorrhea. They reported no significant differences in menstrual blood loss between the 2 groups receiving calcium and calcium + vitamin D and the placebo group (39). This finding is consistent with our results.
The comparison of the frequency of menstrual days indicated no significant differences between the 2 groups before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle). According to the results, the intervention group (LD capsules + vitamin D) showed more normal bleeding duration for a longer period compared to the control group, though this difference was not significantly significant. At the same time, the durations of menstrual bleeding days were normal during and after the intervention in both groups, which was statistically significant.
Based on the literature review, there has been only 1 study conducted on the correlation between vitamin D consumption and menstrual bleeding duration. Hence, we reviewed similar studies that have addressed the relationship between vitamin D and menstrual cycles.
Grandi et al (2022) conducted a prospective experimental study to analyze the effects of vitamin D and green tea extract on the treatment of uterine fibroids among 16 perimenopausal women. The results indicated that receiving epigallocatechin gallate (ie, green tea extract), vitamin D, and vitamin B6 helped significantly reduce the menstrual cycle length (by 0.9 days) among the participants without changing the cycle length and menstrual severity (40).
The finding of the above study is consistent with our results in terms of menstrual duration decline; however, the two studies are not consistent in terms of the menstrual cycle length and menstrual severity. This contradictory result might be explained by the fact that our study analyzed the effect of vitamin D combined with LD capsules, whereas the abovementioned study analyzed the effects of epigallocatechin gallate, vitamin D, and vitamin B6. According to another study, the consumption of vitamin D made the menstrual cycle normal in women with polycystic ovaries (41). The correlation between vitamin D and the menstrual cycle length might be due to the AMH, which helps regulate the use of follicles in ovaries (21). Studies have indicated that vitamin D can regulate AMH signaling in human luteinized granulosa cells. In other words, vitamin D supplements reduce the unnatural AMH serum levels in women with PCOS and vitamin deficiency, indicating the folliculogenesis improvement mechanism of vitamin D (23).
According to the results, there were no significant differences between the control and intervention groups in the frequency of side effects reported by participants.
Tartagni et al (2016) conducted a study on the effects of vitamin D supplements on PMS-caused mood disorders among 158 adolescents with severe vitamin D deficiencies for 4 months. They reported no significant differences between the intervention (vitamin D) and control (placebo) groups in terms of side effects (42). Therefore, taking vitamin D has no effect on increasing or decreasing side effects.
Comparing the patients from the 2 groups in terms of drug satisfaction, the researcher concluded that one-fourth of the control group and half of the intervention group were completely satisfied with the consumed drugs.
Zarei et al (2017) conducted a controlled randomized trial on the effects of calcium + vitamin D and calcium alone on the severity of dysmenorrhea and menstrual blood loss among 85 women aged 18-32 years old with primary dysmenorrhea. The results indicated that 25 participants in the calcium + vitamin D group (86.2%), 21 participants in the calcium group (75%), and 16 participants in the placebo group (57.1%) were satisfied with the treatment (39). Grandi et al (2022) used vitamin D and green tea extract to treat uterine fibroids in 16 premenopausal women and reported very high levels of treatment satisfaction in general (41).
Therefore, receiving vitamin D leads to an increase in patients' satisfaction, which is probably due to the role of vitamin D in reducing menstrual blood loss.
Strength
The strengths of the intervention included preventing possible biases in different ways, such as making participants completely blind, employing a data collector and a data analyst, using only 1 observer (ie, all sampling stages were performed by the researcher) in the analysis, considering different criteria for qualification, following up the intervention to prevent the observer’s biases, cooperating with a reliable gynecologist as the advisor, having the hospital staff deliver the drugs to improve the sense of trust among patients, controlling the effect of blood loss, bleeding duration, and menstrual cycle length in 1 cycle before the intervention within a prospective framework, following up the patients 1 cycle after the intervention to analyze the durability of intervention after its termination, recording side events, measuring the intakes of vitamin D from meals and foods among participants through a questionnaire as a potentially harmful variable, following up participants via phone calls, answering their questions to ensure the correct consumption of capsules, and ensuring the continuation of treatment.
Limitations
Generally, menstrual blood loss is a subjective phenomenon that might vary from person to person. It can also be affected by different factors, such as culture, socioeconomic status, lack of laboratory testing of vitamin D levels before and after the intervention due to financial limitations, the absence of a group that received vitamin D alone, and the prescription of LD pills in the form of capsules.
Conclusion
The results of the statistical tests indicated a significant reduction in the menstrual blood loss of the intervention group (receiving LD capsules combined with vitamin D) compared with the control group (receiving only LD capsules). As the treatment continued, the number of menstrual days and menstrual cycle length improved, resulting in a more normal menstrual cycle in both groups. However, the intervention group (LD capsules + vitamin D) showed more normal bleeding duration and menstrual cycle length for a longer period compared to the control group, though there were no significant differences. As a result, vitamin D can be used along with LD capsules as a supplementary treatment to lessen menstrual bleeding intensity.
Alternative and complementary treatments are becoming increasingly popular (particularly among women) and play a key role in health care systems. People are very interested in these treatments and consider them conventional care services. However, there are different opinions about the quantity and dosage of these treatments. It is hoped that the findings of this study can pave the way for future research on this topic.
Considering the side effects of certain drugs and chemicals, as well as their prohibition in some cases, this method can be considered a less complicated technique for reducing menstrual blood loss among young women in response to AUB-O.
Acknowledgement
The researchers hereby thank all the women participating in this study. We also appreciate the assistance and financial support provided by the Research Department of Tabriz University of Medical Sciences.
Funding sources
The Vice-Chancellor for Research and Technology, Tabriz University of Medical Sciences, has funded the original research.
Ethical statement
IR.TBZMED.REC.1399.1041
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Abnormal uterine bleeding due to ovulatory dysfunction (AUB-O) is defined as excessive, long, and irregular bleeding of the endometrium without any pains or organ-related causes. It is often considered a sign of non-ovulation (1). Menstrual disorders account for 20% of all visits to the gynecologist's office (2). In fact, 35.8% of Iranian women have experienced one or more types of AUB (3). In Iran, a systematic meta-analysis reported the prevalence rates of polymenorrhea, hypermenorrhea, and menorrhagia at 9.94%, 12.94%, and 19.24%, respectively (4).
The complications of AUB-O can cause various psychosocial and physical outcomes, such as embarrassment, fear of unexpected bleeding, low quality of life, anger, unmotivated anxiety and aggression (5), fear of job loss and efficiency decline due to the frequent use of pads and tampons (6), high costs of surgical interventions and use of hygienic care resources (7), anemia of iron deficiency, fatigue, vertigo (8,9), endometrial hyperplasia, and carcinoma (10).
The primary objective of an AUB-O treatment is to stop excessive bleeding and prevent a relapse, whereas the secondary objective is to restart ovulation (10). The primary AUB-O management is the medical treatment, oral contraceptive pills (OCPs) are the preferred treatment for women of reproductive age (11). Other medical treatments include the use of antifibrinolytics, non-steroidal anti-inflammatory drugs (NSAIDs), gonadotropin-releasing hormone (GnRH) agonists, and surgical operation (8).
Despite their high costs and numerous side effects, none of these methods have definitively proven to be effective yet (12). Hence, it is essential to adopt an alternative treatment that could be safe, effective, available, and free of side effects (13).
Many believe in herbal medicines and food supplements, which can be used as natural treatments without any side effects or drug interactions. Many patients now seek alternatives to conventional treatments. Studies have shown that food intake and metabolism might play a key role in causing and treating menstrual disorders (14).
Vitamin D is a fat-soluble vitamin required for the appropriate functioning of the human body (15). The clinical diagnosis of vitamin D deficiency is established by measuring the 25(OH)D serum level (16). According to the definition of the World Health Organization (WHO) and the Institute of Medicine (IOM), a serum level of 25(OH)D of 30 ng/mL (75 nmol/L) or greater is considered sufficient, a level of 20-29 ng/mL (52-72.5 nmol/L) is considered insufficient, and a level of below 20 ng/mL (50 nmol/L) is considered deficient (17).
The biological actions of vitamin D are carried out through the vitamin D receptor (VDR), distributed in different tissues of the body (including the skeleton, parathyroid glands, and reproductive tissues). The vitamin D receptor is expressed in multiple reproductive tissues such as the placenta, uterine, and ovaries (17). According to recent data, vitamin D deficiency might be comorbid with several reproductive disorders, such as uterine fibroids, premature menarche (18), and polycystic ovary syndrome (PCOS) (17). In addition, women with PCOS have reported that vitamin D supplements make menstruation normal and improve the folliculogenesis of ovaries and ovulation (19). According to other studies, dietary vitamin D deficiency can weaken fertility in 2 species of mice (17). In fact, the mice that lack the necessary enzyme to convert the rotational form of vitamin D into its active form have shown signs of estrous cycle disorders, such as stalled follicular growth, prolonged estrous cycle (the period of preparedness for reproduction in female mammals), and lack of ovulation (20).
The relationship between vitamin D and the menstrual cycle length might be due to the anti-Mullerian hormone (AMH), which helps regulate the use of ovarian follicles (21). In fact, AMH is generated in the granulosa cells of growing follicles to hypothetically inhibit the absorption of initial follicles and decelerate the growth of follicles, thereby delaying or preventing atresia and inhibiting the differentiation of granulosa cells (22). Studies have also indicated that vitamin D can regulate AMH signaling in human luteinized granulosa cells. In other words, vitamin D supplements reduce the unnatural AMH serum levels in women with PCOS and vitamin deficiency, indicating the folliculogenesis improvement mechanism of vitamin D (23).
Vitamin D deficiency is among the most prevalent health problems worldwide (24). In fact, the risk of vitamin D deficiency (<30 ng/mL) threatens 69.4% of women in the reproductive age range (25). According to a review study in Iran, vitamin D deficiency rates were reported at 61.90% and 60.45% in non-pregnant and pregnant women, respectively (26). Another review study concluded that women had lower serum levels of this vitamin than men due to their dressing habits (27).
The results of a cross-sectional study indicated that a decreased concentration of 25(OH)D in plasma was associated with an increased chance of an irregular menstruation cycle (28). According to a preliminary study, lower vitamin D levels were correlated with 40% of long menstrual cycles, 27% of oligomenorrhea, and 13% of amenorrhea (29). Another descriptive study reported that increasing the levels of vitamin D would decrease the chances of long menstrual cycles (30). Moreover, a review study reported a strong correlation between vitamin D deficiency and a wide spectrum of polymorphic clinical manifestations, such as menstrual dysfunctions (31).
Having adverse effects on the quality of life, menstrual disorders are highly prevalent among women of productive age. According to the results of reviewing reputable databases of medical references, no study has yet analyzed the effects of combined oral contraceptives (COCs) with and without vitamin D on AUB-O. Given the limited portions of vitamin D in food products, low costs of consuming vitamins, and popularity of vitamins among patients, we decided to study the therapeutic effects of COC capsules with and without vitamin D on menstrual disorders among women of reproductive age.
Methods
The screened people included 102 women of reproductive age, 42 of whom did not meet the inclusion criteria (Flowchart 1).
.PNG)
Study design and sampling
This triple-blind clinical trial was conducted on 60 women of reproductive age (18-45 years old) from June 2021 to February 2022. This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (code: IR.TBZMED.REC.1399.1041), and it was registered at the Iranian Registry of Clinical Trials (IRCT20110606006709N22). Then, sampling permits were granted by the Research Department of Nursing-Midwifery School of Tabriz University of Medical Sciences and Kowsar Hospital in Qazvin.
The convenience sampling method was used to recruit eligible women. Women attending the gynecology clinic were approached by the researcher and given information about the study. Women who expressed interest in participating were asked to complete a checklist of inclusion and exclusion criteria. The target population included the women referring to the gynecology clinic of Kowsar Hospital in Qazvin, Iran. Inclusion criteria were women of reproductive age (18-45 years old) who had been experiencing at least 2 to 3 months of excessive, long, and irregular bleeding (without regular menstrual cycles [28-35 days]) and heaving bleeding for more than 7 days (11). Exclusion criteria were pregnancy, history of any cervix abnormalities (eg, cervical cancer, infection, trauma, polyp, and so on), daily and regular consumption of vitamin D supplements over the past 3 months, consumption of drugs affecting menstrual cycle and bleeding (eg, oral hormonal contraceptives, anticoagulants, selective serotonin reuptake inhibitors, antipsychotics, corticosteroids, hormonal supplements, phenytoin, soy herbal supplements, and ginseng), and any absolute or relative contradictions with combined contraceptive pills, pathological symptoms of the pelvis, pharmaceutical causes, systemic diseases, self-reported unnatural menstruation cycles (ie, irregular cycles followed by prolonged heavy bleeding with a history of such complaints for at least 2 to 3 cycles), and a specialist’s diagnosis at the gynecological clinic.
First, the pregnancy was ruled out by a β-HCG test. Pelvic pathological symptoms were excluded through ultrasonography, endometrial biopsy, Pap test, and pelvic examinations. Patients with a history of thyroid dysfunctions, coagulation disorders, systemic diseases, malignant conditions, and consumption of drugs affecting menstrual cycle and bleeding (eg, oral hormonal contraceptive) over the past 3 months were excluded.
After analyzing the inclusion and exclusion checklists, eligible women were selected and provided with information about the benefits and risks of the study. Participation was voluntary, and the women were asked to sign written consent forms before participating in the study. At the start of the study, each participant was asked to complete a researcher-made questionnaire to obtain accurate information about the menstrual cycle, including cycle length (normal: 21-35 days and abnormal: less than 21 or more than 35 days), bleeding duration (normal: 7 days or less and abnormal: more than 7 days), bleeding volume (using the Higham table), and history of clot expulsion.
All patients were instructed to keep a menstruation calendar. If participants were taking medications that could affect their menstrual cycle or bleeding, they were instructed to provide this information on the questionnaire. Participants were also asked to complete and return a questionnaire gathering personal and demographic information. They were then given the Menstrual Symptoms Questionnaire (MSQ), Higham Questionnaire (to assess bleeding intensity), and the Semi-Quantitative Vitamin D Food Frequency Questionnaire to complete at home for 1 menstrual cycle prior to beginning the intervention.
Tools
1. Demographic Questionnaire: This questionnaire includes different items about age, education, social status, economic status, and some other pieces of information. The participants completed the questionnaire after they entered the research process.
2. Menstrual Symptoms Questionnaire: This questionnaire includes the bleeding period and the menstrual cycle length. It was completed by participants before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle).
3. Higham Questionnaire: The Higham visual chart or the Pictorial Blood Loss Assessment Chart (PBAC) was employed to measure menstrual bleeding. It is an accurate but simple tool for assessing menstrual blood loss through a pictorial chart in which the number of pads and tampons used is recorded by measuring how much blood they are stained with (32). This tool is scored on a specific system, in which a score of 1 is given to a menstrual pad stained with a little blood, a score of 5 is given to a menstrual pad stained with an average amount of blood, and a score of 20 is given to a menstrual pad stained completely with blood. Every small stain (smaller than a circle with a diameter of 2 cm) is scored 1, whereas every wide stain (wider than a circle with a diameter of 3.5 cm) is scored 5. Furthermore, a blood spillage is scored 5. This questionnaire was completed by participants before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle). This chart is a non-laboratory simple method for the diagnosis of menorrhagia. It is a general scoring system that involves observing pads, tampons, and clots appearing as the basis. It is the best tool available for assessing menstrual blood loss, with 86% sensitivity and 89% accuracy (32). Since the Higham chart is valid, there was no need for its retest validity. To determine the reliability, a test-retest was used with 10 days intervals on 20 subjects (r = 0.85).
4. Semi-Quantitative Vitamin D Food Frequency Questionnaire: This questionnaire is completed and analyzed based on the intake of various food portions and their consumption frequency on a daily, weekly, monthly, yearly, “seldom,” or “never” basis. It was used in this study to evaluate the consumption of foods containing vitamin D (33) to make the necessary adjustments between groups in case of differences. For analysis, the daily food intakes (after converting the recorded food into grams) were measured and entered into Nutritionist IV software. The study groups were compared in terms of the mean of those values. To assess reliability, the intraclass correlation coefficient (95% CI) was calculated using the 2-week test-retest method on a sample of 20 subjects, resulting in a coefficient of 0.88 (0.88-0.93).
5. Moreover, the medicine side effect checklist was completed by participants during the intervention (3 cycles), and the satisfaction checklist during 1 cycle of medicine. The medicine side effect checklist included headache, dizziness, visual and nervous disorders, nausea, vomiting, thirst and frequent urination, fatigue, dry mouth, mood changes, breast tenderness, spotting, weight gain, digestive system disorders, and other symptoms.
6. The Checklist of Satisfaction with the Received Medicine: This questionnaire was completed by the participants after the end of the intervention. It included 5 questions ranging from completely satisfied to very dissatisfied.
The content and face validities were then used to determine validity. For this purpose, the questionnaires were given to designated faculty members, and their feedback was collected to make any necessary modifications to the tools. The research tools were standard and have been shown to be reliable in previous relevant studies. Regarding the Higham Questionnaire, Zakherah et al (2011) indicated significant correlations (α = 0.6) between the PBAC scores and the menstrual blood loss measured by the hematinic phosphatase test (used as a gold standard to compare the blood loss from menstrual hygiene products with the venous blood sample) (34).
A randomized block sampling method in blocks of 4 or 6 members was used to assign eligible participants to the control group (ie, the LD group) and intervention group (ie, the LD + vitamin D group) with a 1:1 allocation ratio. An expert who was not part of the research team used Random Allocation Software (RAS) to determine the random allocation sequence, and medication packets numbered 1 to 60 were provided in accordance with this sequence. The researcher, patients, data analyst, and outcome analyst knew nothing about the contents of the packets. The packets that contained the capsules were uniform, sealed, and blurred. Based on the allocation sequence, they were prepared by an individual who was not part of the research team.
The control group received 21 LD capsules (containing 30 µg of ethinylestradiol and 150 µg of levonorgestrel available in Iranian drugstores under serial number 148 by Iran Hormone Pharmaceutical Laboratory) and 9 placebo capsules (containing starch and lactose) in each cycle. The intervention group received 21 combinatory capsules containing vitamin D (1000 units of cholecalciferol; Alborz Daru Company, Qazvin, Iran) and LD (containing 30 µg of ethinylestradiol and 150 µg of levonorgestrel), as well as 9 vitamin D capsules (1000 units of cholecalciferol) to be taken once per day. They were placed separately in similar packets for consumption in 3 cycles. The appearances of vitamin D3, LD, and placebo capsules were altered by dividing the abovementioned drugs into capsule coverings under the supervision of a pharmacist at Alborz Daru Company. In fact, they were given in exactly uniform shapes to the women in the intervention and control groups. To maintain acceptance, the participants were given the drugs for only 1 month and were then informed that they would be given the rest of the drugs again in the next follow-up periods. They were provided with the packets and daily consumption checklist the research questionnaires. They were also told to keep taking 1 capsule per day. The patients were followed up for 3 consecutive cycles during the intervention period and for 1 cycle after completion of the intervention. In addition to the menstruation pattern assessed in the final cycle, drug satisfaction was analyzed using the questionnaires.
One month after the end of the intervention, the researcher collected the questionnaires completed in the final cycle in accordance with the arrangements made via phone calls. Moreover, the participants were followed up every 2 weeks during the intervention period through phone calls.
Sample size
According to Mehrabian et al (35) and based on the mean difference of the 2 independent groups, a 2-tailed test was conducted in G*Power 3.1.2 to assign 25 participants to each group for the menstrual blood loss variable by considering m1 = 18.8, m2 = 15.04, sd1 = 15.04, and sd2 = 4.6 with the power of 80% and α = 0.05. Regarding the number of menstrual days, a 2-tailed test was conducted to assign 17 participants to each group by considering m1 = 9.6, m2 = 7.68, sd1 = 15.04, and sd2 = 1.9 with the power of 80% and α = 0.05. The final sample size was considered 30 participants in each group with respect to a potential attrition rate of 20%.
Statistical methods
After data collection, descriptive statistics, such as frequency (percentage), mean (±SD), and median (25-75 percentile), in addition to analytical statistics (eg, Mann-Whitney U test, Friedman test, Fisher exact test, independent t test, and Cochran Q test) were used in SPSS version 24 (SPSS Inc, Chicago, IL, USA) for data analysis. The normality of the data distribution was checked using the Kolmogorov-Smirnov test. P values less than 0.05 were considered statistically significant.
Results
The baseline characteristics of the participants are shown in Table 1. According to the statistical tests, there were no significant differences between the 2 groups in terms of demographic information and social characteristics of women (P > 0.05; Table 1).
.PNG)
The medians (25-75 percentile) of pre-intervention blood loss were 57 (18-244.5) and 83.5 (40.5-173) in the control and intervention groups, respectively. The post-intervention medians were reported to be 47.5 (33.5-107) and 42.5 (13.5-125) in the control and intervention groups, respectively. According to the Friedman test results, there were no significant differences in the control group before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; P = 0.367) in terms of blood loss. However, there were significant differences in the intervention group in terms of blood loss before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; P = 0.001; Figure 1 and Table 2).
According to the Cochran Q test, there were significant improvements in the control group (P < 0.001) and intervention group (P = 0.002) in terms of the menstrual cycle length during the trial (before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; Table 3).


According to the Cochran Q test, there were significant improvements within the control and intervention groups in the number of menstrual bleeding days during the study (before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle; P < 0.001; Table 4).
The means (±SDs) of vitamin D intakes were 1.368 (±0.628) mg/day and 1.426 (±0.375) mg/day in the control and intervention groups, respectively. The mean difference was 0.058 (95% CI, -0.32 to 0.44). According to the independent t test, there were no significant differences between the study groups in terms of vitamin D intake through diets (P = 0.763).
Less than one-fourth of the control group (16%) and half of the intervention group (52%) were completely satisfied with the consumption of the prescribed drugs, whereas most of the control cases (80%) and nearly half of the intervention cases (48%) were satisfied with the treatment. However, none of the women in the intervention group was satisfied with the treatment. According to the Mann-Whitney U test, there were significant differences between the 2 groups (P = 0.006).
The research results indicated no significant differences between the 2 groups in the frequency of medicinal side effects (eg, headaches [64% vs 60%], vertigo [60% vs 52%], visual and neurological disorders [52% vs 48%], fatigue [84% vs 60%], nausea and vomiting [24% vs 44%], thirst and frequent urination [52% vs 40%], xerostomia [52% vs 60%], mood changes [80% vs 76%], breast sensitivity [36% vs 48%], spotting [44% vs 56%], weight gain [40% vs 44%], gastrointestinal disorders [44% vs 44%], and backaches [4% vs 2%]) in the intervention and control groups, respectively.

Discussion
This study aimed to compare the effect of COC capsules and vitamin D–COC capsules on ovulatory dysfunction in reproductive-age women with AUB-O.
According to the results, menstrual blood loss decreased in the intervention group than in the control group; however, the difference was not significant. Over time, both intervention and control groups showed a significant improvement in bleeding duration and menstrual cycle length, resulting in a more normal menstrual cycle. However, the intervention group showed more normal bleeding duration and menstrual cycle length for a longer period compared to the control group.
The menstrual blood loss comparison results indicated that in both groups, participants experienced less blood loss in each cycle compared to the previous cycle.
In a randomized controlled trial, Adawiyah et al (2020) compared the effect of tranexamic acid and COC on 60 women using depot medroxyprogesterone acetate (DMPA) with AUB, showing that the average length of bleeding was 3.62 ± 5.2 days in the tranexamic acid group and 6.76 ± 9.2 days in the COC group, which was statistically significant (P = 0.018) (36).
In a randomized controlled trial, Sandhya Jain et al (2016) compared the effect of the intravaginal ring and COC on 60 women for 3 consecutive months, showing that blood volume was significantly reduced in both groups; however, although the reduction in Higham scores was higher in the group that received the intravaginal ring, this difference was not statistically significant (P = 0.0286) (37).
Rahmanian et al (2018) conducted a controlled randomized trial on the effect of vitamin D on uterine leiomyoma among 51 women, indicating a significant reduction in the menstrual blood loss of the intervention group (receiving vitamin D) than in the control group (receiving placebo); however, there were no significant differences in the menstrual blood loss between the 2 groups. This finding is consistent with our results (38).
Zarei et al (2017) conducted a controlled randomized clinical study on the effects of calcium + vitamin D and calcium alone on menstrual bleeding among 85 girls aged 18-32 years with primary dysmenorrhea. They reported no significant differences in menstrual blood loss between the 2 groups receiving calcium and calcium + vitamin D and the placebo group (39). This finding is consistent with our results.
The comparison of the frequency of menstrual days indicated no significant differences between the 2 groups before the intervention, during the intervention (3 cycles), and after the intervention (1 cycle). According to the results, the intervention group (LD capsules + vitamin D) showed more normal bleeding duration for a longer period compared to the control group, though this difference was not significantly significant. At the same time, the durations of menstrual bleeding days were normal during and after the intervention in both groups, which was statistically significant.
Based on the literature review, there has been only 1 study conducted on the correlation between vitamin D consumption and menstrual bleeding duration. Hence, we reviewed similar studies that have addressed the relationship between vitamin D and menstrual cycles.
Grandi et al (2022) conducted a prospective experimental study to analyze the effects of vitamin D and green tea extract on the treatment of uterine fibroids among 16 perimenopausal women. The results indicated that receiving epigallocatechin gallate (ie, green tea extract), vitamin D, and vitamin B6 helped significantly reduce the menstrual cycle length (by 0.9 days) among the participants without changing the cycle length and menstrual severity (40).
The finding of the above study is consistent with our results in terms of menstrual duration decline; however, the two studies are not consistent in terms of the menstrual cycle length and menstrual severity. This contradictory result might be explained by the fact that our study analyzed the effect of vitamin D combined with LD capsules, whereas the abovementioned study analyzed the effects of epigallocatechin gallate, vitamin D, and vitamin B6. According to another study, the consumption of vitamin D made the menstrual cycle normal in women with polycystic ovaries (41). The correlation between vitamin D and the menstrual cycle length might be due to the AMH, which helps regulate the use of follicles in ovaries (21). Studies have indicated that vitamin D can regulate AMH signaling in human luteinized granulosa cells. In other words, vitamin D supplements reduce the unnatural AMH serum levels in women with PCOS and vitamin deficiency, indicating the folliculogenesis improvement mechanism of vitamin D (23).
According to the results, there were no significant differences between the control and intervention groups in the frequency of side effects reported by participants.
Tartagni et al (2016) conducted a study on the effects of vitamin D supplements on PMS-caused mood disorders among 158 adolescents with severe vitamin D deficiencies for 4 months. They reported no significant differences between the intervention (vitamin D) and control (placebo) groups in terms of side effects (42). Therefore, taking vitamin D has no effect on increasing or decreasing side effects.
Comparing the patients from the 2 groups in terms of drug satisfaction, the researcher concluded that one-fourth of the control group and half of the intervention group were completely satisfied with the consumed drugs.
Zarei et al (2017) conducted a controlled randomized trial on the effects of calcium + vitamin D and calcium alone on the severity of dysmenorrhea and menstrual blood loss among 85 women aged 18-32 years old with primary dysmenorrhea. The results indicated that 25 participants in the calcium + vitamin D group (86.2%), 21 participants in the calcium group (75%), and 16 participants in the placebo group (57.1%) were satisfied with the treatment (39). Grandi et al (2022) used vitamin D and green tea extract to treat uterine fibroids in 16 premenopausal women and reported very high levels of treatment satisfaction in general (41).
Therefore, receiving vitamin D leads to an increase in patients' satisfaction, which is probably due to the role of vitamin D in reducing menstrual blood loss.
Strength
The strengths of the intervention included preventing possible biases in different ways, such as making participants completely blind, employing a data collector and a data analyst, using only 1 observer (ie, all sampling stages were performed by the researcher) in the analysis, considering different criteria for qualification, following up the intervention to prevent the observer’s biases, cooperating with a reliable gynecologist as the advisor, having the hospital staff deliver the drugs to improve the sense of trust among patients, controlling the effect of blood loss, bleeding duration, and menstrual cycle length in 1 cycle before the intervention within a prospective framework, following up the patients 1 cycle after the intervention to analyze the durability of intervention after its termination, recording side events, measuring the intakes of vitamin D from meals and foods among participants through a questionnaire as a potentially harmful variable, following up participants via phone calls, answering their questions to ensure the correct consumption of capsules, and ensuring the continuation of treatment.
Limitations
Generally, menstrual blood loss is a subjective phenomenon that might vary from person to person. It can also be affected by different factors, such as culture, socioeconomic status, lack of laboratory testing of vitamin D levels before and after the intervention due to financial limitations, the absence of a group that received vitamin D alone, and the prescription of LD pills in the form of capsules.
Conclusion
The results of the statistical tests indicated a significant reduction in the menstrual blood loss of the intervention group (receiving LD capsules combined with vitamin D) compared with the control group (receiving only LD capsules). As the treatment continued, the number of menstrual days and menstrual cycle length improved, resulting in a more normal menstrual cycle in both groups. However, the intervention group (LD capsules + vitamin D) showed more normal bleeding duration and menstrual cycle length for a longer period compared to the control group, though there were no significant differences. As a result, vitamin D can be used along with LD capsules as a supplementary treatment to lessen menstrual bleeding intensity.
Alternative and complementary treatments are becoming increasingly popular (particularly among women) and play a key role in health care systems. People are very interested in these treatments and consider them conventional care services. However, there are different opinions about the quantity and dosage of these treatments. It is hoped that the findings of this study can pave the way for future research on this topic.
Considering the side effects of certain drugs and chemicals, as well as their prohibition in some cases, this method can be considered a less complicated technique for reducing menstrual blood loss among young women in response to AUB-O.
Acknowledgement
The researchers hereby thank all the women participating in this study. We also appreciate the assistance and financial support provided by the Research Department of Tabriz University of Medical Sciences.
Funding sources
The Vice-Chancellor for Research and Technology, Tabriz University of Medical Sciences, has funded the original research.
Ethical statement
IR.TBZMED.REC.1399.1041
Conflict of interest
The authors declare no conflicts of interest.
Author contributions

Type of study: Original Article |
Subject:
Midwifery
References
1. Motta T, Laganà AS, Vitale SG. Dysfunctional Uterine Bleeding. Good Practice in Pediatric and Adolescent Gynecology: Springer; 2018. p.99-115. [View at Publisher] [DOI] [Google Scholar]
2. Vladimirovna SV, Anvarovna SL, Vladimirovna ME, Khidirovna LZJCAJoM, Science N. Menstrual Cycle Disturbances in the Reproductive Period. Central Asian Journal of Medical and Natural Science.2023;4(2):389-97. [View at Publisher] [Google Scholar]
3. Kazemijaliseh H, Tehrani FR, Behboudi-Gandevani S, Khalili D, Hosseinpanah F, Azizi FJAoIM. A Population-Based Study of the Prevalence of Abnormal Uterine Bleeding and its Related Factors among Iranian Reproductive-Age Women: An Updated Data. Archives of Iranian Medicine. 2017;20(9). [View at Publisher] [PMID] [Google Scholar]
4. Samani RO, Hashiani AA, Razavi M, Vesali S, Rezaeinejad M, Maroufizadeh S, et al. The prevalence of menstrual disorders in Iran: A systematic review and meta-analysisInternational Journal of Reproductive BioMedicine. 2018;16(11): 665-78. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Yaşa C, Uğurlucan FGJJocripe. Approach to abnormal uterine bleeding in adolescents. Journal of Clinical Research in Pediatric Endocrinology, 2020;12(Suppl 1):1-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Sriprasert I, Pakrashi T, Kimble T, Archer DFJC, medicine r. Heavy menstrual bleeding diagnosis and medical management. Contraception and Reproductive Medicine. 2017;2(1):1-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Liu Z, Doan QV, Blumenthal P, Dubois RWJVih. A systematic review evaluating health‐related quality of life, work impairment, and health‐care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183-94. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Kaunitz AM, Levine D. Abnormal uterine bleeding in nonpregnant reproductive-age patients: Terminology, evaluation, and approach to diagnosis. 2021. UpToDate. 2023.
9. Jensen JT, Lefebvre P, Laliberté F, Sarda SP, Law A, Pocoski J, et al. Cost burden and treatment patterns associated with management of heavy menstrual bleeding Journal of Women's Health. 2012;21(5):539-47. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Apgar BS, Kaufman AH, George-Nwogu U, Kittendorf ALJAFP. Treatment of menorrhagia. Am Fam Physician. 2007;75(12):1813-9. [View at Publisher] [PMID] [Google Scholar]
11. Berek JS, Novak E. Berek and Novak's gynecology. 16th ed. Philadelphia: Lippincott Williams & Wilkins; 2020. 396-405. [View at Publisher]
12. Kaunitz AM, Levine DJU. Abnormal uterine bleeding in nonpregnant reproductive-age patients: Terminology, evaluation, and approach to diagnosis. UpToDate. 2023. [View at Publisher] [Google Scholar]
13. Geetha B, Shameem I. Efficacy of Unani formulations in the management of dysfunctional uterine bleeding-A randomized controlled trial. International Journal of Medical and Health Research. 2016;2(5):57-61. [View at Publisher] [Google Scholar]
14. Durain DJJom, health ws. Primary dysmenorrhea: assessment and management update. Journal of midwifery & women's health. 2004;49(6):520-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Proctor M, Murphy PJTCL. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Libr. 2005(2).
16. Rafeeq H, Ahmad S, Tareen MB, Shahzad KA, Bashir A, Jabeen R, Shehzadi I. Biochemistry of fat soluble vitamins, sources, biochemical functions and toxicity. Haya: The Saudi Journal of Life Sciences. 2020;5(6):188-96. [View at Publisher] [DOI] [Google Scholar]
17. Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. The Journal of Steroid Biochemistry and Molecular Biology. 2018;175:125-35. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Esmaeili SA, Mohammadian S, Radbakhsh S, Momtazi-Borojeni AA, Parizi KP. AtabatiHadi, MardaniF, Saburi E, Moghaddam AS. Evaluation of vitamin D3 deficiency: a population-based study in northeastern Iran. Journal of Cellular Biochemistry. 2019;120(6):10337-41. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Baird DD, Hill MC, Schectman JM, Hollis BWJE. Vitamin D and risk of uterine fibroids. Epidemiology. 2013;24(3):447. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Rashidi B, Haghollahi F, Shariat M, Zayerii FJTJoO, Gynecology. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol. 2009;48(2):142-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Dicken CL, Israel DD, Davis JB, Sun Y, Shu J, Hardin J, et al. Peripubertal vitamin D3 deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biology of Reproduction. 2012;87(2):51,1-12. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Luk J, Torrealday S, Neal Perry G, Pal LJHr. Relevance of vitamin D in reproduction. Human reproduction. 2012;27(10):3015-27. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Zec I, Tislaric-Medenjak D, Bukovec Megla Z, Kucak IJBm. Anti-Mullerian hormone: a unique biochemical marker of gonadal development and fertility in humans. Biochem Medica. 2011;21(3):219-30. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Irani M, Merhi ZJF, sterility. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertility and Sterility. 2014;102(2):460-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Cashman KDJCti. Vitamin D deficiency: defining, prevalence, causes, and strategies of addressing. Calcified tissue international. 2020;106(1):14-29. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Grzechocińska B, Warzecha D, Szymusik I, Sierdziński J, Wielgoś MJNL. 25 (OH) D serum concentration in women with menstrual disorders-risk factors for vitamin D deficiency. Neuroendocrinology Letters. 2018;39(3).219-25. [View at Publisher] [PMID] [Google Scholar]
27. Tabrizi R, Moosazadeh M, Akbari M, Dabbaghmanesh MH, Mohamadkhani M, Asemi Z, et al. High prevalence of vitamin D deficiency among Iranian population: a systematic review and meta-analysis. Iranian Journal of Medical Sciences. 2018;43(2):125-39. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Lips PJTJosb. Vitamin D status and nutrition in Europe and Asia. Lips PJTJosb, biology m. Vitamin D status and nutrition in Europe and Asia. The Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3-5):620-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Jukic AMZ, Steiner AZ, Baird DDJRB, Endocrinology. Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study. Reproductive Biology and Endocrinology. 2015;13(1):1-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Łagowska KJN. The relationship between vitamin D status and the menstrual cycle in young women: a preliminary study. Nutrients. 2018;10(11):1729. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Jukic AMZ, Upson K, Harmon QE, Baird DDJF, sterility. Increasing serum 25-hydroxyvitamin D is associated with reduced odds of long menstrual cycles in a cross-sectional study of African American women. Fertil Steril. 2016;106(1):172-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Bashmakova N, Lisovskaya T, Vlasova VJGE. Pathogenetic role of vitamin D deficiency in the development of menstrual dysfunction in pubertal girls: a literature review. Gynecol Endocrinol. 2017;33(sup1):52-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Higham JM, O'brien PM, Shaw R. Assessment of menstrual blood loss using a pictorial chart. BJOG: An International Journal of Obstetrics & Gynaecology. 1990 Aug;97(8):734-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, et al. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. The Journal of Nutrition. 2014;144(5):654-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Zakherah MS, Sayed GH, El-Nashar SA, Shaaban MMJG, investigation o. Pictorial blood loss assessment chart in the evaluation of heavy menstrual bleeding: diagnostic accuracy compared to alkaline hematin. Gynecologic and Obstetric Investigation. 2011;71(4):281-4. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Mehrabian F, Abbassi FJPJoMS. Comparing the effects of low-dose contraceptive pills to control dysfunctional uterine bleeding by oral and vaginal methods. Pakistan Journal of Medical Sciences. 2013;29(5):1208. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Adawiyah R, Mulyantoro I, Dewantiningrum J, Pramono NJJoB, Research T. Randomized Controlled Trial of Tranexamic Acid's Effect on Bleeding Length: A Study on DMPA Users with Abnormal Uterine Bleeding Who Receive Low-Dose Oral Contraceptive Pill . Journal of Biomedicine and Translational Research. 2020;6(1):1-5. [View at Publisher] [DOI] [Google Scholar]
38. Jain S, Vaid NB, Narang Y, Suneja A, Guleria KJJoc, JCDR dr. A randomised controlled trial comparing the efficacy and side-effects of intravaginal ring (Nuvaring®) with combined oral hormonal preparation in dysfunctional uterine bleeding. Journal of Clinical and Diagnostic Research. 2016;10(3):QC21. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Rahmanian M, Saghafi M, Darban M, Mirmohammadkhani MJMEJoR, Health. Is Vitamin D Effective in the Treatment of Uterine Leiomyoma? A Randomized Controlled Double-Blind Clinical Trial. Middle East Journal of Rehabilitation and Health. 2018;5(4). [View at Publisher] [DOI] [Google Scholar]
40. Zarei S, Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Javadzadeh Y, Effati-Daryani FJPm. Effects of calcium-Vitamin D and calcium-alone on pain intensity and menstrual blood loss in women with primary dysmenorrhea: A randomized controlled trial. Pain Medicine. 2017;18(1):3-13. [View at Publisher] [DOI] [PMID] [Google Scholar]
41. Grandi G, Del Savio MC, Melotti C, Feliciello L, Facchinetti FJGE. Vitamin D and green tea extracts for the treatment of uterine fibroids in late reproductive life: A pilot, prospective, daily-diary based study. Gynecological Endocrinology. 2022;38(1):63-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
42. Kadoura S, Alhalabi M, Nattouf AHJRJoP, Technology. Effect of calcium and vitamin D supplements as an adjuvant therapy to metformin on lipid profile in vitamin D deficient/insufficient polycystic ovary syndrome patients: A Randomized, Placebo-Controlled Clinical Trial. Advances in Pharmacological and Pharmaceutical Sciences. 2019;12(5):2327-32. [View at Publisher] [DOI] [PMID] [Google Scholar]
43. Tartagni M, Cicinelli MV, Tartagni MV, Alrasheed H, Matteo M, Baldini D, et al. Vitamin D supplementation for premenstrual syndrome-related mood disorders in adolescents with severe hypovitaminosis D. J Pediatr Adolesc Gynecol. 2016;29(4):357-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






