Volume 20, Issue 1 (4-2023)
J Res Dev Nurs Midw 2023, 20(1): 8-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Barati L, Khatami S, Valizadeh M, Khoddam H. Assessing the efficacy of 10% oral dextrose in reducing pain in infants during venipuncture:
A randomized controlled clinical trial. J Res Dev Nurs Midw 2023; 20 (1) :8-10
URL: http://nmj.goums.ac.ir/article-1-1424-en.html
URL: http://nmj.goums.ac.ir/article-1-1424-en.html
1- Neonatal and Children's Health Research Center, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran.
2- Taleghani Hospital, Golestan University of Medical Sciences, Gorgan, Iran.
3- Nursing Research Center, Golestan University of Medical Sciences, Gorgan, Iran. ,khoddam@goums.ac.ir
2- Taleghani Hospital, Golestan University of Medical Sciences, Gorgan, Iran.
3- Nursing Research Center, Golestan University of Medical Sciences, Gorgan, Iran. ,
Full-Text [PDF 483 kb]
(1393 Downloads)
| Abstract (HTML) (4373 Views)
Full-Text: (998 Views)
Introduction
In recent years, infant hospitalizations and subsequently the number of painful procedures have increased (1, 2). Based on studies, invasive medical procedures, especially those requiring the use of needles, are the major source of pain, which may have short-term and long-term physical and developmental consequences for infants (3, 4). Therefore, nursing staff should reduce the number of painful procedures and pay enough attention to pain assessment and management as the 5th vital sign (5). Glucose, sucrose, pacifier, and breast milk are the most common non-pharmacological interventions used before painful procedures (4, 6, 7). The analgesic mechanism of sweet-tasting solutions has not yet been determined (8); however, some studies have shown that activating the opioid system through taste indirectly reduces pain (9). The analgesic effect of sucrose and glucose on neonates has been well established, but little is known about the effect of sweet solutions on procedural pain in infants older than 1 month (2, 4). Despite the greater effectiveness of sucrose solution, 10% dextrose solution is more available in emergency departments and could therefore be a suitable alternative to sucrose solution. For this reason, some studies have been conducted on the effectiveness of dextrose solution for reducing pain in infants (7, 10, 11). In the present study, we aimed to investigate the effectiveness of 10% oral dextrose solution in reducing pain caused by venipuncture in infants.
Methods
The present study was a randomized, double-blind, parallel-group, placebo-controlled clinical trial that was performed on infants aged 1-12 months undergoing venipuncture in the emergency department of Taleghani Hospital in Gorgan, Iran. Inclusion criteria included age of 1-12 months, the need for intravenous catheters for the infant at the time of admission to the emergency department, and parental consent for infants’ participation in the study. Exclusion criteria included impaired level of consciousness, possible poisoning, diabetes, and or other life-threatening illnesses. The sample size of 26 was estimated for the primary outcome using G-power software considering an alpha error of 0.05, a power 80%, and an effect size of 0.5 (Cohen's d). Due to the possibility of loss to follow-up, the sample size of 30 for each group was estimated. The enrollment phase of the study was from October to January. During this period, eligible infants were randomly assigned to intervention and control groups.
The primary outcome of this study was to determine the severity of pain during venipuncture using the Face, Legs, Activity, Cry, and Consolability (FLACC) observational pain assessment scale. This scale evaluates the severity of pain based on the score given for observing five behaviors including facial posture, leg posture, body movements, crying posture, and consolability. According to the behavioral changes after painful stimulation, zero to 2 points are assigned. The total score ranges between 0 and 10, and higher scores indicate greater pain severity (12, 13). Secondary outcomes included the pain score of infants after adjusting for age and gender.
The intervention and control groups were fed 2 ml of 10% dextrose solution and 2 ml of water two minutes before venipuncture, respectively. The syringes were filled by a person other than the one administering them. An experienced nurse performed the venipuncture while the infant was laid down on the bed. During the venipuncture, the behavior of the infants (face and body) was video reordered. All people involved in the study intervention were blind to the content of the syringes and the assignment of infants in the study groups. To maintain the uniformity of the procedure, all venipunctures were done by a fixed nurse.
The recorded videos (60 cases) were observed and scored independently by two nurses blind to the purpose of the study and infants' allocation. Although there was a high agreement between the scorers (Kappa: 0.81, P-value = 0.0001), disagreements were resolved by a third independent reviewer. Data were analyzed using STATA software version 14. One- and two-way ANOVA and ANOVA-ANCOVA tests were used for evaluating the effectiveness of the intervention in reducing infant pain and controlling the effect of potential confounders/covariates such as age and gender. In addition, the independent t-test and Pearson's chi-squared test were used to compare the characteristics of the infants before the intervention. Based on the central limit theorem and the sample size of 30 infants in each group, the distribution of pain scores was considered normal, but the equality of pain scores variances was examined and confirmed before the study (F =0.7129, P= 0.36).
Data were analyzed using statistics (t, f, and chi2) and effect size indices [mean difference, SMD (d Cohen’s), and Partial eta2]. All tests were carried out at 95% confidence interval. Data are presented as mean ± standard deviation (SD). The referred interpretive areas of effect size (Cohen’s d) in this study were: weak effect: 0.20-0.49, moderate effect: 0.50-0.79, and strong effect: 0.80-1.19 (14).
Results
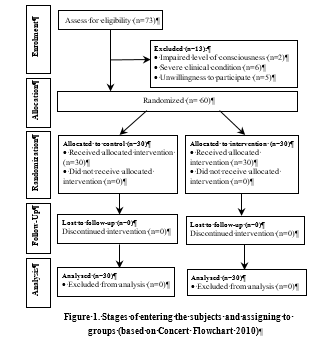
Of 73 infants, 60 infants who had been admitted to the triage department of Taleghani Hospital in Gorgan were eligible to participate Figure 1.
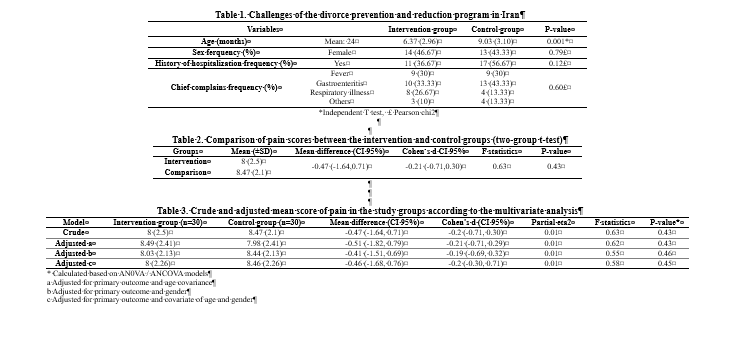
They were mostly girls (46.7%), with a mean (±SD) age of 7.7 (3.29) months. The mean age of the subjects differed significantly between the two groups (Table 1).
The mean pain score in the intervention group was slightly lower than that in the control group (t=0.79, P-value = 0.43). In addition, the effect size (SMD/ Cohen’s d) of 10% oral dextrose on pain reduction was - 0.2 (CI 95%: -0.30 to 0.71) Table 2.
Although there was a significant difference (t= 3.09, P=0.003) in the mean pain score (±SD) between girls and boys [7.30 (2.74) vs. 9 (1.44)], the adjusted analysis for gender showed that the difference in pain score between the groups was non-significant (F=0.55, P=0.46). Adjusted analysis for age showed a non-significant difference between the two groups in terms of pain score (F=0.62, P=0.43) Table 3.
Discussion
This study showed that 2 ml of 10% oral dextrose before venipuncture had a weak and non-significant effect on pain reduction. The majority of studies have been focused on the effectiveness and applicability of sucrose in reducing procedural pain in newborns, while only a limited number of studies have examined the effectiveness of dextrose on infant pain (4, 5, 10, 15). In addition, recommending a fixed concentration of sweet solutions for reducing procedural pain in infants is challenging. Kassab et al. conducted a systematic review on the effectiveness of glucose in reducing needle-induced pain among infants aged less than 12 months. They claimed that because of significant methodological heterogeneity in terms of amount, concentration, and the route of glucose solutions administration as well as the method of outcome assessment, conducting a meta-analysis reach to an inclusive conclusion was not feasible. Therefore, conducting further clinical trials on this issue is required (16). As a result, the lack of enough evidence made it difficult to find similar and appropriate studies to compare our findings with those of previous studies.
Although some studies confirmed the effectiveness of >20% glucose in relieving pain in newborns, there is a need to conduct more studies to find the lowest effective concentration of dextrose with the least complications (e.g. hyperglycemia) (6, 17, 18). In a study by Dilen et al., the effects of different glucose concentrations were assessed on procedure-related pain in infants, and the highest rate of pain reduction in infants was achieved by the administration of 30% glucose solution (19).
Inconsistent with our findings, numerous studies indicated that different concentrations of oral dextrose/glucose solution may reduce pain in neonates and infants following procedures with needles (4, 6, 18, 19, 20). For example, Jatana et al. studied the effects of 10, 25, and 50% oral dextrose and water on pain caused by lancet sampling in 125 infants. They reported that the use of dextrose in different concentrations could exert analgesic effects on full-term neonates with limited side effects (21). In a systematic review and meta-analysis, Harrison et al. recommended oral glucose or sucrose solution for reducing pain and crying duration in infants 1 to 12 months of age undergoing painful procedures (9, 22).
The inconsistency between our findings and the results of other studies can be justified from different aspects. First, it can be due to the difference in pain management procedures. For example, in the study by Harrison et al., the analgesic effect of a sweet solution was assessed for about 3 to 5 minutes with a 2-minute peak, and because intravenous infusion takes longer than vaccination, the effect of the sweet solution may be reduced (23). The second view is the notable variation in the method of pain assessment between different studies (4). For example, the Neonatal Infant Pain Scale, Modified Behavioral Pain Scale, and University of Wisconsin Children's Hospital scales have been used in the mentioned studies, while we used the FLACC scale. The third aspect is the difference in the concentration of dextrose solution used for the interventions. In the present study, a 10% dextrose solution was used, while in most previous studies, a higher concentration of sucrose or glucose was used.
In line with our results, some studies showed that feeding a 10% glucose solution is similar to or slightly better than simple water (18, 20). Considering the research gap, it is recommended to conduct further studies on the effectiveness of sweet solutions, with different amounts and concentrations, in reducing procedural pain in infants older than one month.
Conclusion
The results indicated that using 10% oral dextrose 2 minutes before venipuncture has a weak and non-significant reducing effect on the pain of infants. However, further clinical trials should be conducted on different concentrations and volumes of dextrose solution and incorporate a larger study population.
Acknowledgement
This project (general physician thesis) has been registered on the Iranian clinical trial registration platform (code: IRCT20140820018883N1). The authors would like to thank the Research Deputy of Golestan University of Medical Sciences for the financial and scientific support of this project. In addition, Dr. Abbas Ali Keshtkar is acknowledged for his valuable comments on study design and statistical analysis.
Funding source
This study was supported by the Golestan University of Medical Sciences.
Ethical statement
The study received approval from the Ethics Committee of Goletsan University of Medical Sciences (ethical approval code: IR.GOUMS.REC.1396.268), and written consent was obtained from parents of infants before participation in the study.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this study.
Author contributions
All authors have effective contribution in designing and conducting of the study and also in preparing initial and final version of the manuscript for publication.
In recent years, infant hospitalizations and subsequently the number of painful procedures have increased (1, 2). Based on studies, invasive medical procedures, especially those requiring the use of needles, are the major source of pain, which may have short-term and long-term physical and developmental consequences for infants (3, 4). Therefore, nursing staff should reduce the number of painful procedures and pay enough attention to pain assessment and management as the 5th vital sign (5). Glucose, sucrose, pacifier, and breast milk are the most common non-pharmacological interventions used before painful procedures (4, 6, 7). The analgesic mechanism of sweet-tasting solutions has not yet been determined (8); however, some studies have shown that activating the opioid system through taste indirectly reduces pain (9). The analgesic effect of sucrose and glucose on neonates has been well established, but little is known about the effect of sweet solutions on procedural pain in infants older than 1 month (2, 4). Despite the greater effectiveness of sucrose solution, 10% dextrose solution is more available in emergency departments and could therefore be a suitable alternative to sucrose solution. For this reason, some studies have been conducted on the effectiveness of dextrose solution for reducing pain in infants (7, 10, 11). In the present study, we aimed to investigate the effectiveness of 10% oral dextrose solution in reducing pain caused by venipuncture in infants.
Methods
The present study was a randomized, double-blind, parallel-group, placebo-controlled clinical trial that was performed on infants aged 1-12 months undergoing venipuncture in the emergency department of Taleghani Hospital in Gorgan, Iran. Inclusion criteria included age of 1-12 months, the need for intravenous catheters for the infant at the time of admission to the emergency department, and parental consent for infants’ participation in the study. Exclusion criteria included impaired level of consciousness, possible poisoning, diabetes, and or other life-threatening illnesses. The sample size of 26 was estimated for the primary outcome using G-power software considering an alpha error of 0.05, a power 80%, and an effect size of 0.5 (Cohen's d). Due to the possibility of loss to follow-up, the sample size of 30 for each group was estimated. The enrollment phase of the study was from October to January. During this period, eligible infants were randomly assigned to intervention and control groups.
The primary outcome of this study was to determine the severity of pain during venipuncture using the Face, Legs, Activity, Cry, and Consolability (FLACC) observational pain assessment scale. This scale evaluates the severity of pain based on the score given for observing five behaviors including facial posture, leg posture, body movements, crying posture, and consolability. According to the behavioral changes after painful stimulation, zero to 2 points are assigned. The total score ranges between 0 and 10, and higher scores indicate greater pain severity (12, 13). Secondary outcomes included the pain score of infants after adjusting for age and gender.
The intervention and control groups were fed 2 ml of 10% dextrose solution and 2 ml of water two minutes before venipuncture, respectively. The syringes were filled by a person other than the one administering them. An experienced nurse performed the venipuncture while the infant was laid down on the bed. During the venipuncture, the behavior of the infants (face and body) was video reordered. All people involved in the study intervention were blind to the content of the syringes and the assignment of infants in the study groups. To maintain the uniformity of the procedure, all venipunctures were done by a fixed nurse.
The recorded videos (60 cases) were observed and scored independently by two nurses blind to the purpose of the study and infants' allocation. Although there was a high agreement between the scorers (Kappa: 0.81, P-value = 0.0001), disagreements were resolved by a third independent reviewer. Data were analyzed using STATA software version 14. One- and two-way ANOVA and ANOVA-ANCOVA tests were used for evaluating the effectiveness of the intervention in reducing infant pain and controlling the effect of potential confounders/covariates such as age and gender. In addition, the independent t-test and Pearson's chi-squared test were used to compare the characteristics of the infants before the intervention. Based on the central limit theorem and the sample size of 30 infants in each group, the distribution of pain scores was considered normal, but the equality of pain scores variances was examined and confirmed before the study (F =0.7129, P= 0.36).
Data were analyzed using statistics (t, f, and chi2) and effect size indices [mean difference, SMD (d Cohen’s), and Partial eta2]. All tests were carried out at 95% confidence interval. Data are presented as mean ± standard deviation (SD). The referred interpretive areas of effect size (Cohen’s d) in this study were: weak effect: 0.20-0.49, moderate effect: 0.50-0.79, and strong effect: 0.80-1.19 (14).
Results
Of 73 infants, 60 infants who had been admitted to the triage department of Taleghani Hospital in Gorgan were eligible to participate Figure 1.

They were mostly girls (46.7%), with a mean (±SD) age of 7.7 (3.29) months. The mean age of the subjects differed significantly between the two groups (Table 1).
The mean pain score in the intervention group was slightly lower than that in the control group (t=0.79, P-value = 0.43). In addition, the effect size (SMD/ Cohen’s d) of 10% oral dextrose on pain reduction was - 0.2 (CI 95%: -0.30 to 0.71) Table 2.
Although there was a significant difference (t= 3.09, P=0.003) in the mean pain score (±SD) between girls and boys [7.30 (2.74) vs. 9 (1.44)], the adjusted analysis for gender showed that the difference in pain score between the groups was non-significant (F=0.55, P=0.46). Adjusted analysis for age showed a non-significant difference between the two groups in terms of pain score (F=0.62, P=0.43) Table 3.

Discussion
This study showed that 2 ml of 10% oral dextrose before venipuncture had a weak and non-significant effect on pain reduction. The majority of studies have been focused on the effectiveness and applicability of sucrose in reducing procedural pain in newborns, while only a limited number of studies have examined the effectiveness of dextrose on infant pain (4, 5, 10, 15). In addition, recommending a fixed concentration of sweet solutions for reducing procedural pain in infants is challenging. Kassab et al. conducted a systematic review on the effectiveness of glucose in reducing needle-induced pain among infants aged less than 12 months. They claimed that because of significant methodological heterogeneity in terms of amount, concentration, and the route of glucose solutions administration as well as the method of outcome assessment, conducting a meta-analysis reach to an inclusive conclusion was not feasible. Therefore, conducting further clinical trials on this issue is required (16). As a result, the lack of enough evidence made it difficult to find similar and appropriate studies to compare our findings with those of previous studies.
Although some studies confirmed the effectiveness of >20% glucose in relieving pain in newborns, there is a need to conduct more studies to find the lowest effective concentration of dextrose with the least complications (e.g. hyperglycemia) (6, 17, 18). In a study by Dilen et al., the effects of different glucose concentrations were assessed on procedure-related pain in infants, and the highest rate of pain reduction in infants was achieved by the administration of 30% glucose solution (19).
Inconsistent with our findings, numerous studies indicated that different concentrations of oral dextrose/glucose solution may reduce pain in neonates and infants following procedures with needles (4, 6, 18, 19, 20). For example, Jatana et al. studied the effects of 10, 25, and 50% oral dextrose and water on pain caused by lancet sampling in 125 infants. They reported that the use of dextrose in different concentrations could exert analgesic effects on full-term neonates with limited side effects (21). In a systematic review and meta-analysis, Harrison et al. recommended oral glucose or sucrose solution for reducing pain and crying duration in infants 1 to 12 months of age undergoing painful procedures (9, 22).
The inconsistency between our findings and the results of other studies can be justified from different aspects. First, it can be due to the difference in pain management procedures. For example, in the study by Harrison et al., the analgesic effect of a sweet solution was assessed for about 3 to 5 minutes with a 2-minute peak, and because intravenous infusion takes longer than vaccination, the effect of the sweet solution may be reduced (23). The second view is the notable variation in the method of pain assessment between different studies (4). For example, the Neonatal Infant Pain Scale, Modified Behavioral Pain Scale, and University of Wisconsin Children's Hospital scales have been used in the mentioned studies, while we used the FLACC scale. The third aspect is the difference in the concentration of dextrose solution used for the interventions. In the present study, a 10% dextrose solution was used, while in most previous studies, a higher concentration of sucrose or glucose was used.
In line with our results, some studies showed that feeding a 10% glucose solution is similar to or slightly better than simple water (18, 20). Considering the research gap, it is recommended to conduct further studies on the effectiveness of sweet solutions, with different amounts and concentrations, in reducing procedural pain in infants older than one month.
Conclusion
The results indicated that using 10% oral dextrose 2 minutes before venipuncture has a weak and non-significant reducing effect on the pain of infants. However, further clinical trials should be conducted on different concentrations and volumes of dextrose solution and incorporate a larger study population.
Acknowledgement
This project (general physician thesis) has been registered on the Iranian clinical trial registration platform (code: IRCT20140820018883N1). The authors would like to thank the Research Deputy of Golestan University of Medical Sciences for the financial and scientific support of this project. In addition, Dr. Abbas Ali Keshtkar is acknowledged for his valuable comments on study design and statistical analysis.
Funding source
This study was supported by the Golestan University of Medical Sciences.
Ethical statement
The study received approval from the Ethics Committee of Goletsan University of Medical Sciences (ethical approval code: IR.GOUMS.REC.1396.268), and written consent was obtained from parents of infants before participation in the study.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this study.
Author contributions
All authors have effective contribution in designing and conducting of the study and also in preparing initial and final version of the manuscript for publication.
Type of study: Original Article |
Subject:
Nursing
References
1. Oh SH, Kim IA, Jin H. Optimal dextrose concentration for pain control in healthy newborns during hepatitis B vaccination. Neonatal Medicine. 2018;25(2):72-7. [view at publisher] [DOI] [google scholar]
2. Ranjbar A, Bernstein C, Shariat M, Ranjbar H. Comparison of facilitated tucking and oral dextrose in reducing the pain of heel stick in preterm infants: a randomized clinical trial. BMC pediatrics. 2020; 20:1-9. [view at publisher] [DOI] [google scholar]
3. Birnie KA, Noel M, Chambers CT, Uman LS, Parker JA. Psychological interventions for needle‐related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews. 2018(10):CD005179. [view at publisher] [DOI] [google scholar]
4. Koukou Z, Theodoridou A, Taousani E, Antonakou A, Panteris E, Papadopoulou S-S, et al. Effectiveness of Non-Pharmacological Methods, Such as Breastfeeding, to Mitigate Pain in NICU Infants. Children. 2022;9(10):1568. [view at publisher] [DOI] [google scholar]
5. Gomes PPdS, Lopes APdA, Santos MSNd, Façanha SMdA, Chaves EMC. Non-pharmacological measures for pain relief in venipuncture in newborns: description of behavioral and physiological responses. BrJP. 2019;2(2):142-6. [view at publisher] [DOI] [google scholar]
6. Gouin S, Gaucher N, Lebel D, Desjardins MP. A Randomized Double-Blind Trial Comparing the Effect on Pain of an Oral Sucrose Solution vs. Placebo in Children 1 to 3 Months Old Undergoing Simple Venipuncture. The Journal of emergency medicine. 2018;54(1):33-9. [view at publisher] [DOI] [google scholar]
7. Wade C, Frazer JS, Qian E, Davidson LM, Dash S, te Water Naudé A, et al. Development of locally relevant clinical guidelines for procedure-related neonatal analgesic practice in Kenya: a systematic review and meta-analysis. The Lancet Child & Adolescent Health. 2020;4(10):750-60. [view at publisher] [DOI] [google scholar]
8. Ghaderi F, Ahmadbeigi M, Vossoughi M, Sardarian A. The efficacy of administrating a sweet‐tasting solution for reducing the pain related to dental injections in children: A randomized controlled trial. International Journal of Paediatric Dentistry. 2021;31(2):184-190. [view at publisher] [DOI] [google scholar]
9. Harrison D, Larocque C, Bueno M, Stokes Y, Turner L, Hutton B, et al. Sweet solutions to reduce procedural pain in neonates: a meta-analysis. Pediatrics. 2017;139(1). [view at publisher] [DOI] [google scholar]
10. Stevens B, Yamada J, Campbell-Yeo M, Gibbins S, Harrison D, Dionne K, et al. The minimally effective dose of sucrose for procedural pain relief in neonates: a randomized controlled trial. BMC pediatrics. 2018;18(1):1-8. [view at publisher] [DOI] [google scholar]
11. Trottier ED, Doré-Bergeron M-J, Chauvin-Kimoff L, Baerg K, Ali S. Managing pain and distress in children undergoing brief diagnostic and therapeutic procedures. Paediatrics & child health. 2019;24(8):509-521. [view at publisher] [DOI] [google scholar]
12. Lempinen H, Pölkki T, Kyngäs H, Kaakinen P. Feasibility and Clinical Utility of the Finnish Version of the FLACC Pain Scale in PICU. Journal of Pediatric Nursing. 2020;55:211-16. [view at publisher] [DOI] [google scholar]
13. Crellin DJ, Harrison D, Santamaria N, Huque H, Babl FE. The psychometric properties of the FLACC scale used to assess procedural pain. The Journal of Pain. 2018;19(8):862-72. [view at publisher] [DOI] [google scholar]
14. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdle. Erlbaum. Conner, BE 1988. [view at publisher] [google scholar]
15. Collados‐Gómez L, Ferrera‐Camacho P, Fernandez‐Serrano E, Camacho‐Vicente V, Flores‐Herrero C, García‐Pozo A, et al. Randomised crossover trial showed that using breast milk or sucrose provided the same analgesic effect in preterm infants of at least 28 weeks. Acta Paediatrica. 2018;107(3):436-441. [view at publisher] [DOI] [google scholar]
16. Kassab MI, Roydhouse JK, Fowler C, Foureur M. The Effectiveness of Glucose in Reducing Needle-Related Procedural Pain in Infants. Journal of Pediatric Nursing. 2012;27(1):3-17. [view at publisher] [DOI] [google scholar]
17. Soltani S, Zohoori D, Adineh M. Comparison the effectiveness of breastfeeding, oral 25% dextrose, kangaroo-mother care method, and EMLA cream on pain score level following heal pick sampling in newborns: a randomized clinical trial. Electronic physician. 2018;10(5):6741-6748. [view at publisher] [DOI] [google scholar]
18. Nikrouz L, Rostami S, Alijani Renani H, Rasekh A, Naghizadeh MM. Comparing the Effect of Breast-Feeding and Oral Glucose on Infants Vaccination Pain. JABS. 2014;4(2): 225-232. [view at publisher] [google scholar]
19. Dilen B, Elseviers M. Oral glucose solution as pain relief in newborns: results of a clinical trial. Birth. 2010;37(2):98-105. [view at publisher] [DOI] [google scholar]
20. Nayak R, Nagaraj KN, Gururaj G. Prevention of Pain During Screening for Retinopathy of Prematurity: A Randomized Control Trial Comparing Breast Milk, 10% Dextrose and Sterile Water. The Indian Journal of Pediatrics. 2020;87(5):353-8. [view at publisher] [DOI] [google scholar]
21. Jatana S, Dalal S, Wilson C. Analgesic effect of oral glucose in neonates. Medical Journal Armed Forces India. 2003;59(2):100-104. [view at publisher] [DOI] [google scholar]
22. Harrison D, Larocque C, Reszel J, Harrold J, Aubertin C, Dowling D, et al. Be Sweet to Babies during painful procedures. Advances in Neonatal Care. 2017;17(5):372-80. [view at publisher] [DOI] [google scholar]
23. Harrison D, Stevens B, Bueno M, Yamada J, Adams-Webber T, Beyene J, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Archives of disease in childhood. 2010;95(6):406-13. [view at publisher] [DOI] [google scholar]
24. Friedrichsdorf SJ, Goubert L. Pediatric pain treatment and prevention for hospitalized children. Der Schmerz. 2021;35:195-210. [view at publisher] [DOI] [google scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






