Volume 22, Issue 2 (6-2025)
J Res Dev Nurs Midw 2025, 22(2): 44-51 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Majdi F, Farshbaf-Khalili A, Mirzayi M, Hakimi P, Hajizadeh K, Shahnazi M. Comparing the effects of nettle extract with combined oral contraceptives on improving clinical and paraclinical symptoms of polycystic Ovary Syndrome: A triple-blind randomized controlled clinical trial. J Res Dev Nurs Midw 2025; 22 (2) :44-51
URL: http://nmj.goums.ac.ir/article-1-1981-en.html
URL: http://nmj.goums.ac.ir/article-1-1981-en.html
Faride Majdi1 

 , Azizeh Farshbaf-Khalili2
, Azizeh Farshbaf-Khalili2 

 , Mohammadreza Mirzayi3
, Mohammadreza Mirzayi3 

 , Parvin Hakimi4
, Parvin Hakimi4 

 , Khadije Hajizadeh5
, Khadije Hajizadeh5 

 , Mahnaz Shahnazi1
, Mahnaz Shahnazi1 




 , Azizeh Farshbaf-Khalili2
, Azizeh Farshbaf-Khalili2 

 , Mohammadreza Mirzayi3
, Mohammadreza Mirzayi3 

 , Parvin Hakimi4
, Parvin Hakimi4 

 , Khadije Hajizadeh5
, Khadije Hajizadeh5 

 , Mahnaz Shahnazi1
, Mahnaz Shahnazi1 


1- Department of Midwifery, Faculty of Nursing and Midwifery, Tabriz University of Medical sciences, Qazvin, Iran
2- Physical Medicine and Rehabilitation Research Centre, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3- Department of Traditional Medicine, School of Persian Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4- Women’s Reproductive Health Research Center, Tabriz Universitu of Medical Sciences, Tabriz, Iran
5- Department of Midwifery, Faculty of Nursing and Midwifery, Tabriz University of Medical sciences, Qazvin, Iran ,hajizade_k@yahoo.com
2- Physical Medicine and Rehabilitation Research Centre, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3- Department of Traditional Medicine, School of Persian Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4- Women’s Reproductive Health Research Center, Tabriz Universitu of Medical Sciences, Tabriz, Iran
5- Department of Midwifery, Faculty of Nursing and Midwifery, Tabriz University of Medical sciences, Qazvin, Iran ,
Keywords: Nettle extract, Polycystic Ovary Syndrome, Contraceptives, Oral, Combined, Signs and Symptoms
Full-Text [PDF 671 kb]
(225 Downloads)
| Abstract (HTML) (332 Views)
Results
The demographic and obstetric characteristics are presented in Table 1. According to the statistical results, there was no significant difference between the study and control groups, except for the education level and the number of pregnancies (Table 1).
Based on the ANCOVA test, after controlling for baseline values and the variables of education and number of pregnancies, there was no significant statistical difference between the two groups regarding serum levels of dehydroepiandrosterone (DHEA) (Mean difference: -18.68 μg/dL, 95% confidence interval: -47.31 to 9.97, P = 0.197), total serum testosterone (Mean difference: 1.88 ng/dL, 95% confidence interval: -1.34 to 1.88, P = 0.24), follicle-stimulating hormone (FSH) (Mean difference: 0.268 IU/L, 95% confidence interval: -1.60 to 1.23, P = 0.549), and luteinizing hormone (LH) (Mean difference: -2.91 IU/L, 95% confidence interval: -3.92 to 9.73, P = 0.398). However, the two groups showed a significant statistical difference in fasting blood glucose levels (Mean difference: -3.13 mg/dL, 95% confidence interval: -5.75 to -0.52, P = 0.020) (Table 2).
After the intervention, the mean (Standard deviation) cycle length in the nettle extract group was 38.43 (11.66) days, and in the control group, it was 31.00 (5.79) days (Mean difference: 5.20, 95% confidence interval: 0.49 to 9.91, P = 0.031). Based on the ANCOVA test, while controlling for baseline values, education, and the number of pregnancies, a significant statistical difference between the two groups was noted for this measure. However, after the intervention, the duration of menstrual bleeding in the nettle extract group remained 6.43 (1.8) days, and in the control group, it remained 6.30 (2.2) days. The ANCOVA test, with baseline values, education, and the number of pregnancies controlled, indicated no significant statistical difference between the two groups for this measure (Mean difference: 0.08, 95% confidence interval: -0.70 to 0.72, P = 0.982) (Table 3).
Discussion
This study aimed to compare the effects of nettle root extract with COCs on improving the clinical and paraclinical symptoms of PCOS. A randomized, controlled, triple-blind trial was conducted.
The results showed that after the intervention, there was no significant statistical difference between the serum levels of DHEA and total testosterone in the nettle extract group compared to the control group. The nettle extract and the COCs were equally effective in reducing these hormones.
In alignment with the goal of this study, a clinical trial conducted by Najafipour et al. (2014) aimed to evaluate the therapeutic effects of nettle in women with hyperandrogenism. In that study, total and free testosterone and DHEA serum levels were measured before and after the intervention. Patients were treated for 4 months with either standard treatment (Control group) or dried nettle root extract (Intervention group). The results showed a significant reduction in total and free testosterone levels in the nettle group post-treatment (41).
Regarding the aim of comparing the mean serum levels of gonadotropins (FSH and LH) in the intervention and control groups, the results indicated that after the intervention, there was no significant statistical difference in the mean serum levels of FSH and LH between the nettle extract group and the control group. Both nettle extract and COCs were equally effective in reducing these hormones.
Although no human studies were found directly related to this specific objective, a survey by Esfedeh and colleagues (2019) investigated the effect of nettle extract as a metformin supplement on ovarian tissue in a diabetic rat model. At the end of the treatment, the effects of metformin and nettle root extract on ovarian tissue and biochemical factors, such as blood glucose levels and sex hormones, were compared. After 4 weeks of treatment with metformin and nettle root extract, hyperglycemia and body weight reduction showed improvement. The simultaneous administration of metformin and nettle root extract resulted in a significant increase in primary and secondary follicles, as well as an increase in corpus luteum size, a reduction in atretic follicles, and a significant rise in the levels of FSH, LH, and testosterone, compared to metformin alone. This study concluded that nettle root, with its antioxidant properties and other bioactive components, can complement metformin, improving blood sugar levels and ovarian dysfunctions. Studies conducted in diabetic rats support the hypothesis that antioxidant compounds can ameliorate this pathogenesis, potentially due to their direct effects on insulin secretion, prevention of beta-cell death, or regulation of beta-cell proliferation (42).
The results showed that after the intervention, the mean (Standard deviation) fasting blood glucose levels in the participants of the nettle extract group had a statistically significant difference compared to the control group, indicating that the use of nettle extract affected fasting blood glucose levels. In a double-blind clinical trial conducted by Kianbakht et al. (2013), the effect of nettle leaf extract on controlling blood glucose in patients with advanced type 2 diabetes was investigated. The intervention group consisted of 46 patients who took 500 mg of nettle capsules orally every 8 hours for 3 months, while the control group received a placebo capsule under the same conditions. No side effects, including hypoglycemia, were reported. At the end of the study, nettle extract significantly reduced fasting glucose, postprandial glucose (2 hours after meals), and HbA1c levels. These results demonstrate the positive effects of nettle extract on blood glucose control in patients with type 2 diabetes (43).
Three potential mechanisms for the effects of nettle in lowering blood glucose include: 1) the impact of nettle on muscle cells by increasing the formation of permeable pores, which enhances glucose uptake in muscles, ultimately leading to a reduction in blood glucose levels in type 2 diabetes. 2) Stimulating the release of insulin from beta cells. 3) The influence on carbohydrate hydrolysis inhibitors. Compounds (flavonoids, peptides, and amines) found in the nettle plant leaves are known to have anti-diabetic effects (44). However, a study by Esfedegh et al. (2019) aimed at determining the impact of nettle extract as a supplement to metformin on ovarian tissue in a diabetic rat model demonstrated that the antioxidant properties of nettle are attributed to its flavonoid compounds (42).
Regarding the goal of normalizing menstrual cycles in both groups, the results showed that COCs were more effective than nettle extract in regulating the intervals between menstrual cycles. Additionally, the study results indicated that nettle extract was as effective as COCs in reducing the duration of menstrual bleeding.
A study conducted by Najafipour and colleagues (2014) aimed to determine the effect of nettle on women with hyperandrogenism. It showed that the improvement in menstrual regularity for patients receiving cyproterone compound and spironolactone was significantly higher compared to the nettle group (41). The study also showed that after the intervention, the mean hirsutism score in the participants of the nettle extract group did not differ significantly from that of the control group, and the nettle extract was as effective as COCs in reducing hormones. Although no study directly measuring the effects of nettle on hirsutism was found, a study by Najafipour and colleagues (2014), aimed at determining the impact of nettle on women with hyperandrogenism, showed that the improvement in skin conditions such as acne and oily skin was significantly greater in the group receiving the cyproterone compound and spironolactone compared to the nettle group (41).
After the intervention, most participants in both groups experienced moderate bleeding; however, there was a significant difference in the amount of bleeding between the two groups. Specifically, there were three cases of heavy bleeding in the nettle group, whereas no heavy bleeding was reported in the combined oral contraceptive group. The effect of COCs in reducing bleeding was greater than that of nettle extract. No similar study was found based on the search conducted using scientific sources.
A strength of the study was its design as a triple-blind study, with only one observer conducting all sampling stages to assess inclusion criteria and monitor the intervention, thereby preventing observer bias. However, the study has several limitations: the short duration of the intervention and the lack of evaluation of long-term effects. It did not assess the impact of different doses of nettle extract and was conducted with the lowest possible dose. Higher doses might have shown more significant effects. Additionally, confounding variables such as diet, insulin or lipid levels and exercise habits were not considered. Therefore, it is recommended that the impact of different doses of nettle extract on PCOS symptoms, potential dose-response relationships, and optimal therapeutic doses be examined. It is also recommended to investigate the possible synergistic effects of nettle extract alongside other established PCOS treatments, including metformin or lifestyle changes.
Conclusion
The results of the research indicated that nettle extract was as effective as oral contraceptives (LD pills) in reducing levels of dehydroepiandrosterone (DHEA), total testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), as well as in decreasing the hirsutism score and menstrual bleeding duration. Therefore, the use of nettle, which is an easy, inexpensive, and accessible method, can be considered an adjunctive treatment alongside other therapy. Overall, this study contributes to the body of scientific evidence related to the management of PCOS and emphasizes the importance of precise and controlled clinical trials in evaluating potential therapeutic interventions. Further research in this field is essential to address the unmet clinical needs of women with PCOS and to advance the development of evidence-based management strategies for this common endocrine disorder. The findings of this study can be utilized in educational, clinical, and research contexts.
Acknowledgement
We appreciate the Clinical Research Development Unit of Al-Zahra Hospital, Tabriz University of Medical Sciences, for their research consultation and language editing services. Thank you for your valuable support and assistance. Additionally, we would like to express our sincere gratitude to all the women who voluntarily participated in this study.
Funding sources
The Vice-Chancellor for Research and Technology, Tabriz University of Medical Sciences, has funded the original research.
Ethical statement
The study was conducted after obtaining the necessary approvals from the Research Ethics Committee of Tabriz University of Medical Sciences (TBZMED.REC.1402.113.IR) and registration in the Iranian Clinical Trial Registry (IRCT20110606006709N25). All ethical principles in human research and clinical trials were adhered to in accordance with the ethical codes of research and the principles outlined in the Helsinki Declaration. This included voluntary participation with informed written consent, the right to withdraw from the study, confidentiality of participants' identities, and the avoidance of any harm to participants.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
Study conceptualization: F.M, Kh.H, M.Sh. Study design: Kh.H, A.F-Kh, M.Sh, MR.M. Data collection: F.M, P.H, MR.M. Statistical analysis: Kh.H, M.Sh, A.F-Kh. Manuscript preparation: Kh.H, M.Sh, A.F-Kh. Manuscript editing: Kh.H, M.Sh, F.M. Project administration: F.M, M.Sh, Kh.H. All authors read and approved the final version of the manuscript.
Data availability statement
Data will be made available upon reasonable request from the corresponding author, subject to review and agreement by the research team.
Full-Text: (41 Views)
Introduction
Polycystic Ovary Syndrome (PCOS) is the most common endocrine reproductive disorder worldwide. The prevalence of PCOS according to the diagnostic criteria of the NIH, Rotterdam and AE-PCOS Society were 13.6, 19.4, and 17.8, respectively (1). PCOS is characterized by chronic oligo- or anovulation, polycystic ovarian morphology, clinical or biochemical signs of hyperandrogenism, and is often associated with metabolic disturbances-primarily insulin resistance and compensatory hyperinsulinemia (2,3).
PCOS is a significant contributor to female infertility and other various metabolic disorders. It has been reported as the cause of infertility in 70% of affected women. PCOS was diagnosed in three out of every ten infertile women (4). Women with PCOS face a higher risk of miscarriage (5,6) and pregnancy complications, including gestational diabetes, hypertension, and preterm labor (6). Additionally, the risk of developing endometrial hyperplasia and endometrial cancer is up to four times higher compared to women without PCOS (7,8). Cardiovascular disorders, such as dyslipidemia, hypertension, and obstructive sleep apnea, are also more prevalent in this syndrome (9,10). Due to distress caused by hyperandrogenic symptoms (Such as acne, hirsutism, alopecia, and male-pattern baldness), weight gain, and infertility, women with PCOS often experience mood disorders (11,12).
The Rotterdam diagnostic criteria are commonly used worldwide to diagnose PCOS (13). When menstrual cycles are irregular or absent, a diagnosis of PCOS should be considered, as approximately 85-90% of women with oligomenorrhea and 30-40% of women with amenorrhea are affected by PCOS (14).
Lifestyle interventions, including a combination of a healthy diet and increased physical activity (15,16), as well as the use of combined oral contraceptive pills (COCPs), are recommended as the first-line medical treatment for managing hyperandrogenism and regulating menstrual cycles in women with PCOS (17,18). Medications such as metformin and anti-androgens like flutamide and spironolactone are also effective in managing the condition (19,20).
Women with PCOS may have contraindications to the use of oral contraceptive pills (OCPs) (21). Although ovulation induction with clomiphene has been successful, pregnancy rates remain inexplicably low (22). Up to 30% of women with PCOS-particularly those who are overweight-do not respond to clomiphene treatment (22-24). Metformin is effective in improving insulin sensitivity and hyperandrogenism; however, its use is often associated with a high incidence of side effects, including nausea, vomiting, and gastrointestinal disturbances (25).
The World Health Organization recommends using herbal medicines and encourages researchers to incorporate medicinal plants into traditional medicine practices for their preventive, therapeutic, and rehabilitative roles (26). Herbal treatments may serve as highly effective therapeutic options for PCOS due to their fewer side effects compared to chemical medications (27). Nettle (Urtica dioica) is a perennial herbaceous plant belonging to the Urticaceae family. It grows in temperate and tropical wastelands worldwide and is distributed across Iran's northern, northwestern, and central regions. The plant includes about 60 genera and 700 species (28) and is classified as a key medicinal plant in the European Pharmacopoeia. Nettle possesses several medicinal properties, including antioxidant (29), anti-inflammatory, anti-ulcer (30), anticancer (31), and antibacterial and antifungal effects (32).
Flavonoids, tannins, scopoletin, sterols, fatty acids, polysaccharides, isolectins, and sterols are phytochemicals reported from this plant. Due to its ease of collection, wide availability, and significant biological activities, nettle has been transformed into medicine and food in many countries, particularly in the Mediterranean region (28). Nettle extract is effective in controlling morphological and histological changes in polycystic ovaries, as well as complications related to metabolic syndrome and alterations in sex hormones in a mouse model of PCOS (33).
In studies conducted on rats, mice, dogs, chickens, and cell culture models, the effects of nettle extract have been shown to control inflammatory cytokines, clinical symptoms, immune responses, blood glucose levels, glucose transporter gene expression, and lipid peroxidation in various organs (34,35). Nettle's ability to regulate lipid profiles and enhance insulin sensitivity is believed to alleviate some common symptoms of metabolic syndrome and type 2 diabetes in PCOS, an effect attributed to its flavonoid compounds (36).
Given the side effects associated with chemical medications in the treatment of PCOS, the use of herbal remedies with therapeutic effects and fewer adverse reactions is expanding (37). Based on a search of electronic databases, aside from one animal study, no human research has been found regarding the effects of nettle extract on the clinical and paraclinical symptoms of PCOS in women. Considering the high prevalence of PCOS among women of reproductive age and its negative impact on their quality of life, this study was conducted to evaluate the effects of nettle extract compared to combined oral contraceptives (COCs) on the clinical and paraclinical manifestations of PCOS in affected women.
Methods
Study design and sampling
This study was a triple-blind randomized controlled trial, involving participants, researchers, and data analysts who were all unaware of the type of intervention. It employed a parallel design and was conducted between December 2022 and April 2025, following the acquisition of the necessary approvals from the Research Ethics Committee. The study included 60 women (n = 30 per group) aged 18 to 45 years who visited the clinics of Tabriz University of Medical Sciences and the Faculty of Traditional Medicine in Iran. Participants and were diagnosed with PCOS according to the Rotterdam criteria, which require the presence of two out of three criteria: clinical or biochemical hyperandrogenism, menstrual cycle disturbances, or polycystic ovarian ultrasound findings. Eligible participants were selected by the researcher based on the inclusion criteria. The objectives and procedures of the study were explained, and written informed consent was obtained from those willing to participate. Additionally, participants completed demographic and menstrual cycle questionnaires, which were recorded in a checklist.
The primary outcomes of this study included levels of hirsutism. In contrast, the secondary outcomes encompassed paraclinical criteria such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), fasting blood sugar (FBS), and testosterone levels, as well as clinical criteria including menstrual cycle intervals, duration, and bleeding intensity.
The inclusion criteria for this study were as follows: a confirmed diagnosis of PCOS according to the Rotterdam criteria; women aged 18 to 45 years; the ability to read and write; a body mass index between 18.5 and 30; no use of hormonal contraceptive medications; any other hormonal drugs currently or vitamin and minerals supplements within the past three months; and no desire for pregnancy. The exclusion criteria included both relative and absolute contraindications for low-dose (LD) pills, such as having a blood pressure above 140/90 mmHg, pregnancy or breastfeeding, alcohol and tobacco use, suspicion of breast cancer, coronary heart disease, or endometrial carcinoma. Additionally, women undergoing infertility treatment at the time of the study, those with a history of previous surgery on one or both ovaries, allergies to nettle, pregnancy and those with disorders that cause hyperandrogenism (Such as Cushing's syndrome, adrenal hyperplasia, androgen-producing tumors) were also excluded.
Participants were randomly allocated into two groups (Intervention and control) using block randomization with block sizes of 4 to 6 and a 1:1 allocation ratio. The allocation sequence was determined by a person not involved in the study, using the RAS (Random Allocation Software) version 1.0.0. Participants in both groups received education on a healthy lifestyle (Nutrition and physical activity) from the researcher, and they were instructed and emphasized not to attempt pregnancy during the treatment period and to use a reliable non-hormonal contraceptive method.
To conceal the drug allocation, the treatments or controls were placed in opaque envelopes, sequentially numbered from 1 to 60. This preparation was carried out according to the allocation sequence by an individual not involved in the study. The intervention lasted for 3 months. Participants in the intervention group received 500 mg nettle extract capsules (Containing flavonoids, tannins, and sterols), taking one capsule daily (30 capsules in total), which were prepared and packaged by Sina Novandish Company in Tabriz, Iran. Subsequently, the Pharmaceutical Research Department of the Faculty of Pharmacy analyzed and confirmed the contents. Participants in the control group received LD capsules (Containing 30 micrograms of ethinylestradiol and 150 micrograms of levonorgestrel), which included 21 active capsules and nine placebo capsules for a 30-day cycle. These were packaged and numbered identically to the nettle capsules (Ensuring the participant would begin with the LD capsules and finish with the placebo). These capsules were also prepared by Sina Novandish Company. To ensure blinding between the study and control groups, 30 nettle or LD capsules were placed in similar containers. The LD tablets were housed inside the capsules, with 21 of the capsules being green and nine oranges. To minimize gastrointestinal side effects, the medication was taken at night after dinner. Only a one-month supply was provided to promote adherence, with participants instructed to obtain the remaining medication in subsequent visits. The envelopes, along with a daily medication checklist and a side effects log, were supplied.
Blood samples were drawn from the brachial vein before the intervention, after 8-hour fasting period, using a 5 mL syringe. The samples were placed in test tubes and immediately sent to the laboratory. Following centrifugation at 3000 rpm, the serum samples were transferred to 1.5 mL microtubes and stored at -70°C until analysis. In this study, serum levels of blood tests were measured using the enzyme-linked immunosorbent assay (ELISA) method at the provincial laboratory. To assess the reliability of the instrument, a separate sample of five women was prepared and provided to the experimenter under two different names, without disclosing the study group designation. The results were analyzed using the Pearson correlation coefficient, which yielded a correlation coefficient of r = 0.89, indicating a strong level of correlation.
A daily medication checklist was provided to participants to assess drug adherence, allowing them to mark off each dose taken. They were also asked to bring their medication packets to each visit to check the number of doses consumed and remaining. Additionally, a side effect report form was given to them to record any adverse effects after taking the medication. The potential side effects were fully explained to the participants, and they were advised to notify the researcher if they experienced any. Every night, a reminder to take the medication was sent via SMS, and a weekly phone call was made to encourage participants to stay in the study. At the end of week 12, the hirsutism scale and menstrual disturbance questionnaires were completed again, and blood samples were taken.
Sample size
The sample size was calculated using G-Power software (Version 3.1.9.4) based on the study by Attarzadeh (38) related to dehydroepiandrosterone )DHEA(, with M1 = 2204 and assuming a 10% reduction due to the intervention (M2 = 1763), with SD1 = SD2 = 640, α = 0.05, and 80% power, the sample size was calculated to be 27 participants. Considering a potential 10% dropout rate, the final sample size was adjusted to 30 participants per group. Given the higher sample size based on the DHEA variable, the final sample size for this study was determined to be 30 participants in each group.
Data collection tools
Sociodemographic questionnaire: This questionnaire included questions about age, marital status, occupation, monthly income, education, living conditions, number of pregnancies, number of deliveries, number of living children, number of miscarriages, and history of infertility.
Menstrual cycle profile questionnaire: This questionnaire addressed menstrual cycle status, including questions about the intervals, duration, and amount of menstrual bleeding over the past three months. It was completed at the beginning of the study and again three months after the intervention. The Higam Chart (Higham, 1990) was used to assess bleeding volume. This visual tool evaluates the amount of menstrual blood loss, assigning a score of 1 for a sanitary pad with slight blood staining, 5 for a half-soaked pad, and 20 for a completely soaked pad. Clots received scores from 1 to 5 based on their size. The chart records menstruation days in the horizontal row and stained pads according to their saturation level in the vertical row. Three levels of bleeding severity (Mild, moderate, and severe) were marked for each corresponding day. At the end of menstruation, each symbol was multiplied by its score to calculate the total score. A score below 50 indicated mild bleeding, between 51 and 100 indicated moderate bleeding, and above 100 indicated heavy bleeding during menstruation. This standard questionnaire does not require validity determination (39).
Hirsutism questionnaire: The Ferriman-Gallwey score was used to evaluate hirsutism before and three months after the intervention. This scale assesses hair growth in nine body (Chin, sternum, thigh, scapula, arm, pubis, upper lip, abdomen, upper sacrum) areas and is scored on a Likert scale from 0 to 4, where 0 means no hair and 4 means excessive hair growth. The maximum score for an individual was 36, and the minimum score was 0 (40). This standard questionnaire does not require validity determination and was tested for reliability using inter-rater reliability (Kappa=0.89).
Adverse event checklist: This checklist records any adverse events that occurred during the study and their severity.
Daily medication consumption checklist: The daily medication and side effects form was filled out each day to ensure that pills were taken and to track any side effects, completed within 30 minutes after taking the medication.
Data analysis
The Kolmogorov-Smirnov test was used to check the normality of the data. The Independent T-test and Chi-square test were used to compare demographic characteristics. The Independent T-test was applied to compare the mean hirsutism scores, laboratory values, menstrual cycle frequency, and duration before the intervention. After the intervention, ANCOVA (Analysis of Covariance) was used with baseline values as a covariate (Education and number of pregnancies). The Chi-square test was used to compare menstrual bleeding between two groups. A p-value of <0.05 was considered statistically significant. The data were analyzed using SPSS software version 26. The analysis approach was based on Intention to Treat (ITT).
A total of 86 participants were evaluated, with 16 excluded based on the established criteria. Ten individuals met the inclusion criteria but withdrew from the study due to lack of consent. Therefore, 60 women aged 18 to 45 years with PCOS who met all the inclusion criteria were enrolled after obtaining informed consent. They were randomly assigned to either the intervention or control group. No participants were lost to follow-up during the third month, and after 12 weeks, 60 participants were re-evaluated (Figure 1).
Polycystic Ovary Syndrome (PCOS) is the most common endocrine reproductive disorder worldwide. The prevalence of PCOS according to the diagnostic criteria of the NIH, Rotterdam and AE-PCOS Society were 13.6, 19.4, and 17.8, respectively (1). PCOS is characterized by chronic oligo- or anovulation, polycystic ovarian morphology, clinical or biochemical signs of hyperandrogenism, and is often associated with metabolic disturbances-primarily insulin resistance and compensatory hyperinsulinemia (2,3).
PCOS is a significant contributor to female infertility and other various metabolic disorders. It has been reported as the cause of infertility in 70% of affected women. PCOS was diagnosed in three out of every ten infertile women (4). Women with PCOS face a higher risk of miscarriage (5,6) and pregnancy complications, including gestational diabetes, hypertension, and preterm labor (6). Additionally, the risk of developing endometrial hyperplasia and endometrial cancer is up to four times higher compared to women without PCOS (7,8). Cardiovascular disorders, such as dyslipidemia, hypertension, and obstructive sleep apnea, are also more prevalent in this syndrome (9,10). Due to distress caused by hyperandrogenic symptoms (Such as acne, hirsutism, alopecia, and male-pattern baldness), weight gain, and infertility, women with PCOS often experience mood disorders (11,12).
The Rotterdam diagnostic criteria are commonly used worldwide to diagnose PCOS (13). When menstrual cycles are irregular or absent, a diagnosis of PCOS should be considered, as approximately 85-90% of women with oligomenorrhea and 30-40% of women with amenorrhea are affected by PCOS (14).
Lifestyle interventions, including a combination of a healthy diet and increased physical activity (15,16), as well as the use of combined oral contraceptive pills (COCPs), are recommended as the first-line medical treatment for managing hyperandrogenism and regulating menstrual cycles in women with PCOS (17,18). Medications such as metformin and anti-androgens like flutamide and spironolactone are also effective in managing the condition (19,20).
Women with PCOS may have contraindications to the use of oral contraceptive pills (OCPs) (21). Although ovulation induction with clomiphene has been successful, pregnancy rates remain inexplicably low (22). Up to 30% of women with PCOS-particularly those who are overweight-do not respond to clomiphene treatment (22-24). Metformin is effective in improving insulin sensitivity and hyperandrogenism; however, its use is often associated with a high incidence of side effects, including nausea, vomiting, and gastrointestinal disturbances (25).
The World Health Organization recommends using herbal medicines and encourages researchers to incorporate medicinal plants into traditional medicine practices for their preventive, therapeutic, and rehabilitative roles (26). Herbal treatments may serve as highly effective therapeutic options for PCOS due to their fewer side effects compared to chemical medications (27). Nettle (Urtica dioica) is a perennial herbaceous plant belonging to the Urticaceae family. It grows in temperate and tropical wastelands worldwide and is distributed across Iran's northern, northwestern, and central regions. The plant includes about 60 genera and 700 species (28) and is classified as a key medicinal plant in the European Pharmacopoeia. Nettle possesses several medicinal properties, including antioxidant (29), anti-inflammatory, anti-ulcer (30), anticancer (31), and antibacterial and antifungal effects (32).
Flavonoids, tannins, scopoletin, sterols, fatty acids, polysaccharides, isolectins, and sterols are phytochemicals reported from this plant. Due to its ease of collection, wide availability, and significant biological activities, nettle has been transformed into medicine and food in many countries, particularly in the Mediterranean region (28). Nettle extract is effective in controlling morphological and histological changes in polycystic ovaries, as well as complications related to metabolic syndrome and alterations in sex hormones in a mouse model of PCOS (33).
In studies conducted on rats, mice, dogs, chickens, and cell culture models, the effects of nettle extract have been shown to control inflammatory cytokines, clinical symptoms, immune responses, blood glucose levels, glucose transporter gene expression, and lipid peroxidation in various organs (34,35). Nettle's ability to regulate lipid profiles and enhance insulin sensitivity is believed to alleviate some common symptoms of metabolic syndrome and type 2 diabetes in PCOS, an effect attributed to its flavonoid compounds (36).
Given the side effects associated with chemical medications in the treatment of PCOS, the use of herbal remedies with therapeutic effects and fewer adverse reactions is expanding (37). Based on a search of electronic databases, aside from one animal study, no human research has been found regarding the effects of nettle extract on the clinical and paraclinical symptoms of PCOS in women. Considering the high prevalence of PCOS among women of reproductive age and its negative impact on their quality of life, this study was conducted to evaluate the effects of nettle extract compared to combined oral contraceptives (COCs) on the clinical and paraclinical manifestations of PCOS in affected women.
Methods
Study design and sampling
This study was a triple-blind randomized controlled trial, involving participants, researchers, and data analysts who were all unaware of the type of intervention. It employed a parallel design and was conducted between December 2022 and April 2025, following the acquisition of the necessary approvals from the Research Ethics Committee. The study included 60 women (n = 30 per group) aged 18 to 45 years who visited the clinics of Tabriz University of Medical Sciences and the Faculty of Traditional Medicine in Iran. Participants and were diagnosed with PCOS according to the Rotterdam criteria, which require the presence of two out of three criteria: clinical or biochemical hyperandrogenism, menstrual cycle disturbances, or polycystic ovarian ultrasound findings. Eligible participants were selected by the researcher based on the inclusion criteria. The objectives and procedures of the study were explained, and written informed consent was obtained from those willing to participate. Additionally, participants completed demographic and menstrual cycle questionnaires, which were recorded in a checklist.
The primary outcomes of this study included levels of hirsutism. In contrast, the secondary outcomes encompassed paraclinical criteria such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), fasting blood sugar (FBS), and testosterone levels, as well as clinical criteria including menstrual cycle intervals, duration, and bleeding intensity.
The inclusion criteria for this study were as follows: a confirmed diagnosis of PCOS according to the Rotterdam criteria; women aged 18 to 45 years; the ability to read and write; a body mass index between 18.5 and 30; no use of hormonal contraceptive medications; any other hormonal drugs currently or vitamin and minerals supplements within the past three months; and no desire for pregnancy. The exclusion criteria included both relative and absolute contraindications for low-dose (LD) pills, such as having a blood pressure above 140/90 mmHg, pregnancy or breastfeeding, alcohol and tobacco use, suspicion of breast cancer, coronary heart disease, or endometrial carcinoma. Additionally, women undergoing infertility treatment at the time of the study, those with a history of previous surgery on one or both ovaries, allergies to nettle, pregnancy and those with disorders that cause hyperandrogenism (Such as Cushing's syndrome, adrenal hyperplasia, androgen-producing tumors) were also excluded.
Participants were randomly allocated into two groups (Intervention and control) using block randomization with block sizes of 4 to 6 and a 1:1 allocation ratio. The allocation sequence was determined by a person not involved in the study, using the RAS (Random Allocation Software) version 1.0.0. Participants in both groups received education on a healthy lifestyle (Nutrition and physical activity) from the researcher, and they were instructed and emphasized not to attempt pregnancy during the treatment period and to use a reliable non-hormonal contraceptive method.
To conceal the drug allocation, the treatments or controls were placed in opaque envelopes, sequentially numbered from 1 to 60. This preparation was carried out according to the allocation sequence by an individual not involved in the study. The intervention lasted for 3 months. Participants in the intervention group received 500 mg nettle extract capsules (Containing flavonoids, tannins, and sterols), taking one capsule daily (30 capsules in total), which were prepared and packaged by Sina Novandish Company in Tabriz, Iran. Subsequently, the Pharmaceutical Research Department of the Faculty of Pharmacy analyzed and confirmed the contents. Participants in the control group received LD capsules (Containing 30 micrograms of ethinylestradiol and 150 micrograms of levonorgestrel), which included 21 active capsules and nine placebo capsules for a 30-day cycle. These were packaged and numbered identically to the nettle capsules (Ensuring the participant would begin with the LD capsules and finish with the placebo). These capsules were also prepared by Sina Novandish Company. To ensure blinding between the study and control groups, 30 nettle or LD capsules were placed in similar containers. The LD tablets were housed inside the capsules, with 21 of the capsules being green and nine oranges. To minimize gastrointestinal side effects, the medication was taken at night after dinner. Only a one-month supply was provided to promote adherence, with participants instructed to obtain the remaining medication in subsequent visits. The envelopes, along with a daily medication checklist and a side effects log, were supplied.
Blood samples were drawn from the brachial vein before the intervention, after 8-hour fasting period, using a 5 mL syringe. The samples were placed in test tubes and immediately sent to the laboratory. Following centrifugation at 3000 rpm, the serum samples were transferred to 1.5 mL microtubes and stored at -70°C until analysis. In this study, serum levels of blood tests were measured using the enzyme-linked immunosorbent assay (ELISA) method at the provincial laboratory. To assess the reliability of the instrument, a separate sample of five women was prepared and provided to the experimenter under two different names, without disclosing the study group designation. The results were analyzed using the Pearson correlation coefficient, which yielded a correlation coefficient of r = 0.89, indicating a strong level of correlation.
A daily medication checklist was provided to participants to assess drug adherence, allowing them to mark off each dose taken. They were also asked to bring their medication packets to each visit to check the number of doses consumed and remaining. Additionally, a side effect report form was given to them to record any adverse effects after taking the medication. The potential side effects were fully explained to the participants, and they were advised to notify the researcher if they experienced any. Every night, a reminder to take the medication was sent via SMS, and a weekly phone call was made to encourage participants to stay in the study. At the end of week 12, the hirsutism scale and menstrual disturbance questionnaires were completed again, and blood samples were taken.
Sample size
The sample size was calculated using G-Power software (Version 3.1.9.4) based on the study by Attarzadeh (38) related to dehydroepiandrosterone )DHEA(, with M1 = 2204 and assuming a 10% reduction due to the intervention (M2 = 1763), with SD1 = SD2 = 640, α = 0.05, and 80% power, the sample size was calculated to be 27 participants. Considering a potential 10% dropout rate, the final sample size was adjusted to 30 participants per group. Given the higher sample size based on the DHEA variable, the final sample size for this study was determined to be 30 participants in each group.
Data collection tools
Sociodemographic questionnaire: This questionnaire included questions about age, marital status, occupation, monthly income, education, living conditions, number of pregnancies, number of deliveries, number of living children, number of miscarriages, and history of infertility.
Menstrual cycle profile questionnaire: This questionnaire addressed menstrual cycle status, including questions about the intervals, duration, and amount of menstrual bleeding over the past three months. It was completed at the beginning of the study and again three months after the intervention. The Higam Chart (Higham, 1990) was used to assess bleeding volume. This visual tool evaluates the amount of menstrual blood loss, assigning a score of 1 for a sanitary pad with slight blood staining, 5 for a half-soaked pad, and 20 for a completely soaked pad. Clots received scores from 1 to 5 based on their size. The chart records menstruation days in the horizontal row and stained pads according to their saturation level in the vertical row. Three levels of bleeding severity (Mild, moderate, and severe) were marked for each corresponding day. At the end of menstruation, each symbol was multiplied by its score to calculate the total score. A score below 50 indicated mild bleeding, between 51 and 100 indicated moderate bleeding, and above 100 indicated heavy bleeding during menstruation. This standard questionnaire does not require validity determination (39).
Hirsutism questionnaire: The Ferriman-Gallwey score was used to evaluate hirsutism before and three months after the intervention. This scale assesses hair growth in nine body (Chin, sternum, thigh, scapula, arm, pubis, upper lip, abdomen, upper sacrum) areas and is scored on a Likert scale from 0 to 4, where 0 means no hair and 4 means excessive hair growth. The maximum score for an individual was 36, and the minimum score was 0 (40). This standard questionnaire does not require validity determination and was tested for reliability using inter-rater reliability (Kappa=0.89).
Adverse event checklist: This checklist records any adverse events that occurred during the study and their severity.
Daily medication consumption checklist: The daily medication and side effects form was filled out each day to ensure that pills were taken and to track any side effects, completed within 30 minutes after taking the medication.
Data analysis
The Kolmogorov-Smirnov test was used to check the normality of the data. The Independent T-test and Chi-square test were used to compare demographic characteristics. The Independent T-test was applied to compare the mean hirsutism scores, laboratory values, menstrual cycle frequency, and duration before the intervention. After the intervention, ANCOVA (Analysis of Covariance) was used with baseline values as a covariate (Education and number of pregnancies). The Chi-square test was used to compare menstrual bleeding between two groups. A p-value of <0.05 was considered statistically significant. The data were analyzed using SPSS software version 26. The analysis approach was based on Intention to Treat (ITT).
A total of 86 participants were evaluated, with 16 excluded based on the established criteria. Ten individuals met the inclusion criteria but withdrew from the study due to lack of consent. Therefore, 60 women aged 18 to 45 years with PCOS who met all the inclusion criteria were enrolled after obtaining informed consent. They were randomly assigned to either the intervention or control group. No participants were lost to follow-up during the third month, and after 12 weeks, 60 participants were re-evaluated (Figure 1).
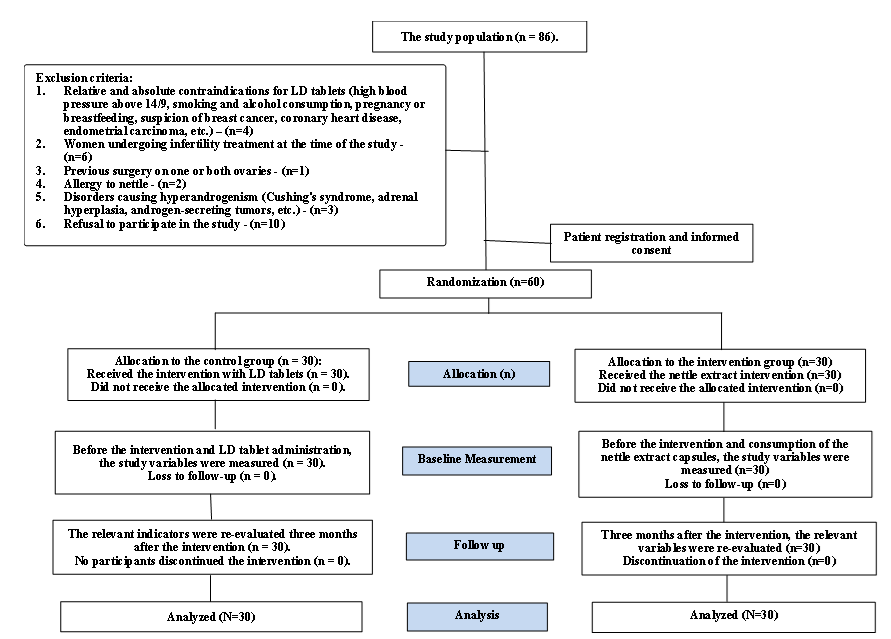 Figure 1. Study flowchart |
Results
The demographic and obstetric characteristics are presented in Table 1. According to the statistical results, there was no significant difference between the study and control groups, except for the education level and the number of pregnancies (Table 1).
Based on the ANCOVA test, after controlling for baseline values and the variables of education and number of pregnancies, there was no significant statistical difference between the two groups regarding serum levels of dehydroepiandrosterone (DHEA) (Mean difference: -18.68 μg/dL, 95% confidence interval: -47.31 to 9.97, P = 0.197), total serum testosterone (Mean difference: 1.88 ng/dL, 95% confidence interval: -1.34 to 1.88, P = 0.24), follicle-stimulating hormone (FSH) (Mean difference: 0.268 IU/L, 95% confidence interval: -1.60 to 1.23, P = 0.549), and luteinizing hormone (LH) (Mean difference: -2.91 IU/L, 95% confidence interval: -3.92 to 9.73, P = 0.398). However, the two groups showed a significant statistical difference in fasting blood glucose levels (Mean difference: -3.13 mg/dL, 95% confidence interval: -5.75 to -0.52, P = 0.020) (Table 2).
After the intervention, the mean (Standard deviation) cycle length in the nettle extract group was 38.43 (11.66) days, and in the control group, it was 31.00 (5.79) days (Mean difference: 5.20, 95% confidence interval: 0.49 to 9.91, P = 0.031). Based on the ANCOVA test, while controlling for baseline values, education, and the number of pregnancies, a significant statistical difference between the two groups was noted for this measure. However, after the intervention, the duration of menstrual bleeding in the nettle extract group remained 6.43 (1.8) days, and in the control group, it remained 6.30 (2.2) days. The ANCOVA test, with baseline values, education, and the number of pregnancies controlled, indicated no significant statistical difference between the two groups for this measure (Mean difference: 0.08, 95% confidence interval: -0.70 to 0.72, P = 0.982) (Table 3).
|
Table 1. Comparison of socio-demographic characteristics between study groups
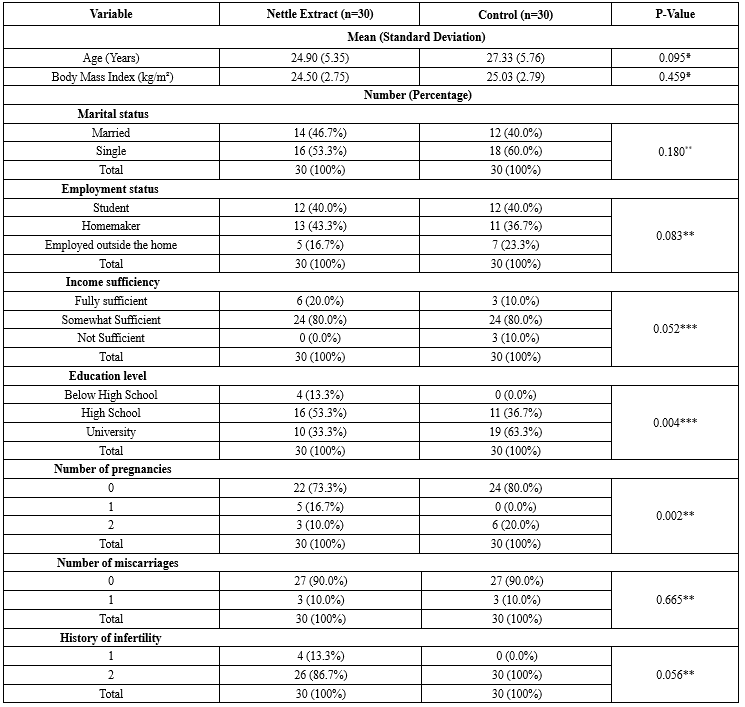 *Independent Samples T-test *Chi-square test **Chi-square test for trend |
After the intervention, the mean (Standard deviation) hirsutism score in participants from the nettle extract group was 6.7 (4.48), while in the control group, it was 6.83 (4.64). Based on the ANCOVA test, which controlled for baseline values and the variables of education and number of pregnancies, there was no statistically significant difference between the groups (Mean difference: -0.49, 95% confidence interval: -1.81 to 1.64, P = 0.149) (Table 3).
The majority of participants in both groups showed moderate bleeding before the intervention, with 21 participants (70.0%) in the nettle extract group and 24 participants (80.0%) in the control group. According to the chi-square test, there was no statistically significant difference between the groups (P = 0.296). After the intervention, most participants continued to experience moderate bleeding-26 participants (86.7%) in the control group and 27 participants (90.0%) in the nettle extract group-while severe bleeding was noted in 3 cases within the nettle extract group. In contrast, no severe bleeding occurred in the control group. The chi-square test indicated a statistically significant difference between the groups (P = 0.008) (Table 4).
Half of the participants in the nettle extract group (16 participants, 52.4%) and the control group (16 participants, 52.4%) reported relative satisfaction with the medication. According to the Mann-Whitney test, this difference between the two groups was not statistically significant (P = 0.360).
According to the patients' statements, no side effects were reported in either the study or control groups, and all participants had taken the LD and nettle capsules according to the routine.
The majority of participants in both groups showed moderate bleeding before the intervention, with 21 participants (70.0%) in the nettle extract group and 24 participants (80.0%) in the control group. According to the chi-square test, there was no statistically significant difference between the groups (P = 0.296). After the intervention, most participants continued to experience moderate bleeding-26 participants (86.7%) in the control group and 27 participants (90.0%) in the nettle extract group-while severe bleeding was noted in 3 cases within the nettle extract group. In contrast, no severe bleeding occurred in the control group. The chi-square test indicated a statistically significant difference between the groups (P = 0.008) (Table 4).
Half of the participants in the nettle extract group (16 participants, 52.4%) and the control group (16 participants, 52.4%) reported relative satisfaction with the medication. According to the Mann-Whitney test, this difference between the two groups was not statistically significant (P = 0.360).
According to the patients' statements, no side effects were reported in either the study or control groups, and all participants had taken the LD and nettle capsules according to the routine.
|
Table 2. Comparison of the mean serum levels of DHEA, total testosterone, fasting blood sugar, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) between study groups
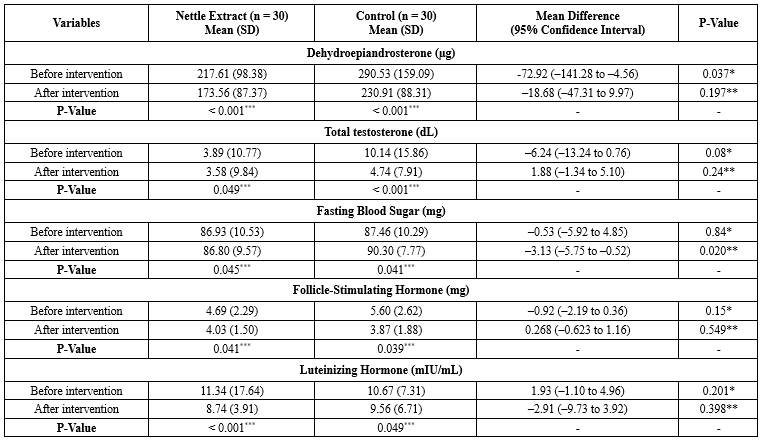 * Independent sample t-test **ANCOVA test controlling for baseline values and the variables of education and number of pregnancies *** Paired sample t-test Table 3. Comparison of menstrual cycle intervals, duration of bleeding, and hirsutism score between study groups 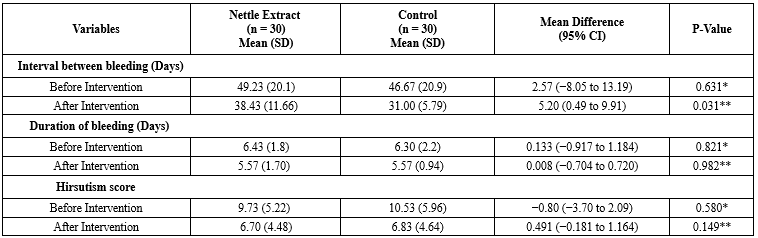 * Independent sample t-test **ANCOVA test controlling for baseline values and the variables of education and number of pregnancies |
|
Table 4. Comparison of bleeding intensity based on the Higham chart between study groups
 *Chi-square test |
Discussion
This study aimed to compare the effects of nettle root extract with COCs on improving the clinical and paraclinical symptoms of PCOS. A randomized, controlled, triple-blind trial was conducted.
The results showed that after the intervention, there was no significant statistical difference between the serum levels of DHEA and total testosterone in the nettle extract group compared to the control group. The nettle extract and the COCs were equally effective in reducing these hormones.
In alignment with the goal of this study, a clinical trial conducted by Najafipour et al. (2014) aimed to evaluate the therapeutic effects of nettle in women with hyperandrogenism. In that study, total and free testosterone and DHEA serum levels were measured before and after the intervention. Patients were treated for 4 months with either standard treatment (Control group) or dried nettle root extract (Intervention group). The results showed a significant reduction in total and free testosterone levels in the nettle group post-treatment (41).
Regarding the aim of comparing the mean serum levels of gonadotropins (FSH and LH) in the intervention and control groups, the results indicated that after the intervention, there was no significant statistical difference in the mean serum levels of FSH and LH between the nettle extract group and the control group. Both nettle extract and COCs were equally effective in reducing these hormones.
Although no human studies were found directly related to this specific objective, a survey by Esfedeh and colleagues (2019) investigated the effect of nettle extract as a metformin supplement on ovarian tissue in a diabetic rat model. At the end of the treatment, the effects of metformin and nettle root extract on ovarian tissue and biochemical factors, such as blood glucose levels and sex hormones, were compared. After 4 weeks of treatment with metformin and nettle root extract, hyperglycemia and body weight reduction showed improvement. The simultaneous administration of metformin and nettle root extract resulted in a significant increase in primary and secondary follicles, as well as an increase in corpus luteum size, a reduction in atretic follicles, and a significant rise in the levels of FSH, LH, and testosterone, compared to metformin alone. This study concluded that nettle root, with its antioxidant properties and other bioactive components, can complement metformin, improving blood sugar levels and ovarian dysfunctions. Studies conducted in diabetic rats support the hypothesis that antioxidant compounds can ameliorate this pathogenesis, potentially due to their direct effects on insulin secretion, prevention of beta-cell death, or regulation of beta-cell proliferation (42).
The results showed that after the intervention, the mean (Standard deviation) fasting blood glucose levels in the participants of the nettle extract group had a statistically significant difference compared to the control group, indicating that the use of nettle extract affected fasting blood glucose levels. In a double-blind clinical trial conducted by Kianbakht et al. (2013), the effect of nettle leaf extract on controlling blood glucose in patients with advanced type 2 diabetes was investigated. The intervention group consisted of 46 patients who took 500 mg of nettle capsules orally every 8 hours for 3 months, while the control group received a placebo capsule under the same conditions. No side effects, including hypoglycemia, were reported. At the end of the study, nettle extract significantly reduced fasting glucose, postprandial glucose (2 hours after meals), and HbA1c levels. These results demonstrate the positive effects of nettle extract on blood glucose control in patients with type 2 diabetes (43).
Three potential mechanisms for the effects of nettle in lowering blood glucose include: 1) the impact of nettle on muscle cells by increasing the formation of permeable pores, which enhances glucose uptake in muscles, ultimately leading to a reduction in blood glucose levels in type 2 diabetes. 2) Stimulating the release of insulin from beta cells. 3) The influence on carbohydrate hydrolysis inhibitors. Compounds (flavonoids, peptides, and amines) found in the nettle plant leaves are known to have anti-diabetic effects (44). However, a study by Esfedegh et al. (2019) aimed at determining the impact of nettle extract as a supplement to metformin on ovarian tissue in a diabetic rat model demonstrated that the antioxidant properties of nettle are attributed to its flavonoid compounds (42).
Regarding the goal of normalizing menstrual cycles in both groups, the results showed that COCs were more effective than nettle extract in regulating the intervals between menstrual cycles. Additionally, the study results indicated that nettle extract was as effective as COCs in reducing the duration of menstrual bleeding.
A study conducted by Najafipour and colleagues (2014) aimed to determine the effect of nettle on women with hyperandrogenism. It showed that the improvement in menstrual regularity for patients receiving cyproterone compound and spironolactone was significantly higher compared to the nettle group (41). The study also showed that after the intervention, the mean hirsutism score in the participants of the nettle extract group did not differ significantly from that of the control group, and the nettle extract was as effective as COCs in reducing hormones. Although no study directly measuring the effects of nettle on hirsutism was found, a study by Najafipour and colleagues (2014), aimed at determining the impact of nettle on women with hyperandrogenism, showed that the improvement in skin conditions such as acne and oily skin was significantly greater in the group receiving the cyproterone compound and spironolactone compared to the nettle group (41).
After the intervention, most participants in both groups experienced moderate bleeding; however, there was a significant difference in the amount of bleeding between the two groups. Specifically, there were three cases of heavy bleeding in the nettle group, whereas no heavy bleeding was reported in the combined oral contraceptive group. The effect of COCs in reducing bleeding was greater than that of nettle extract. No similar study was found based on the search conducted using scientific sources.
A strength of the study was its design as a triple-blind study, with only one observer conducting all sampling stages to assess inclusion criteria and monitor the intervention, thereby preventing observer bias. However, the study has several limitations: the short duration of the intervention and the lack of evaluation of long-term effects. It did not assess the impact of different doses of nettle extract and was conducted with the lowest possible dose. Higher doses might have shown more significant effects. Additionally, confounding variables such as diet, insulin or lipid levels and exercise habits were not considered. Therefore, it is recommended that the impact of different doses of nettle extract on PCOS symptoms, potential dose-response relationships, and optimal therapeutic doses be examined. It is also recommended to investigate the possible synergistic effects of nettle extract alongside other established PCOS treatments, including metformin or lifestyle changes.
Conclusion
The results of the research indicated that nettle extract was as effective as oral contraceptives (LD pills) in reducing levels of dehydroepiandrosterone (DHEA), total testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), as well as in decreasing the hirsutism score and menstrual bleeding duration. Therefore, the use of nettle, which is an easy, inexpensive, and accessible method, can be considered an adjunctive treatment alongside other therapy. Overall, this study contributes to the body of scientific evidence related to the management of PCOS and emphasizes the importance of precise and controlled clinical trials in evaluating potential therapeutic interventions. Further research in this field is essential to address the unmet clinical needs of women with PCOS and to advance the development of evidence-based management strategies for this common endocrine disorder. The findings of this study can be utilized in educational, clinical, and research contexts.
Acknowledgement
We appreciate the Clinical Research Development Unit of Al-Zahra Hospital, Tabriz University of Medical Sciences, for their research consultation and language editing services. Thank you for your valuable support and assistance. Additionally, we would like to express our sincere gratitude to all the women who voluntarily participated in this study.
Funding sources
The Vice-Chancellor for Research and Technology, Tabriz University of Medical Sciences, has funded the original research.
Ethical statement
The study was conducted after obtaining the necessary approvals from the Research Ethics Committee of Tabriz University of Medical Sciences (TBZMED.REC.1402.113.IR) and registration in the Iranian Clinical Trial Registry (IRCT20110606006709N25). All ethical principles in human research and clinical trials were adhered to in accordance with the ethical codes of research and the principles outlined in the Helsinki Declaration. This included voluntary participation with informed written consent, the right to withdraw from the study, confidentiality of participants' identities, and the avoidance of any harm to participants.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
Study conceptualization: F.M, Kh.H, M.Sh. Study design: Kh.H, A.F-Kh, M.Sh, MR.M. Data collection: F.M, P.H, MR.M. Statistical analysis: Kh.H, M.Sh, A.F-Kh. Manuscript preparation: Kh.H, M.Sh, A.F-Kh. Manuscript editing: Kh.H, M.Sh, F.M. Project administration: F.M, M.Sh, Kh.H. All authors read and approved the final version of the manuscript.
Data availability statement
Data will be made available upon reasonable request from the corresponding author, subject to review and agreement by the research team.
Type of study: Original Article |
Subject:
Midwifery
References
1. Farhadi-Azar M, Behboudi-Gandevani S, Rahmati M, Mahboobifard F, Khalili Pouya E, Ramezani Tehrani F, et al. The Prevalence of Polycystic Ovary Syndrome, Its Phenotypes and Cardio-Metabolic Features in a Community Sample of Iranian Population: Tehran Lipid and Glucose Study. Front Endocrinol (Lausanne). 2022;13:825528. [View at publisher] [DOI] [PMID] [Google Scholar]
2. Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016;37(5):467-520. [View at publisher] [DOI] [PMID] [Google Scholar]
3. Houston EJ, Templeman NM. Reappraising the relationship between hyperinsulinemia and insulin resistance in PCOS. Endocrinol. 2025;265(2):e240269. [View at publisher] [DOI] [PMID] [Google Scholar]
4. Alam Z, Alseari S, Alameemi M, Alzaabi M, Alkhoori R, Östlundh L,et al. Prevalence of polycystic ovary syndrome among infertile women in the Gulf Cooperation Council (GCC) countries: A systematic review and meta-analysis. Heliyon. 2024;10(24):e40603. [View at publisher] [DOI] [PMID] [Google Scholar]
5. Bui LM, Aghajanova L, B. Lathi R, Sokalska A. Polycystic ovary syndrome and miscarriage: a narrative review. F&S Reviews. 2024.5(4): 100078. [View at Publisher] [DOI] [Google Scholar]
6. Vanky E, S. Løvvik T. Polycystic Ovary Syndrome and Pregnancy - From a Clinical Perspective. Curr Opin Endocr Metab Res. 2020;12:8-13. [View at publisher] [DOI] [Google Scholar]
7. Charalampakis V, Tahrani AA, Helmy A, Gupta JK, Singhal R. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstet Gynecol Reprod Biol. 2016;207:220-6. [View at publisher] [DOI] [PMID] [Google Scholar]
8. Xue Z, Li J, Feng J, Han H, Zhao J, Zhang J, et al. Research Progress on the Mechanism Between Polycystic Ovary Syndrome and Abnormal Endometrium. Front Physiol. 2021;12:788772. [View at publisher] [DOI] [PMID] [Google Scholar]
9. Guan C, Zahid S, Minhas AS, Ouyang P, Vaught A, Baker VL,et al. Polycystic ovary syndrome: a "risk-enhancing " factor for cardiovascular disease. Fertil Steril. 2022;117(5):924-35. [View at publisher] [DOI] [PMID] [Google Scholar]
10. Sam S, Tasali E. Role of obstructive sleep apnea in metabolic risk in PCOS. Curr Opin Endocr Metab Res. 2021;17:46-51. [View at publisher] [DOI] [PMID] [Google Scholar]
11. Maliszewska B, Tokarzewska A, Łasica M, Małyszek M, Łaba L, Dziurda S, et al. Psychiatric disorders in women with polycystic ovary syndrome. J Pre Clin Clin Res. 2024;18(3):286-92. [View at publisher] [DOI] [Google Scholar]
12. Sukhapure M, Eggleston K, Fenton A, Frampton C, Porter RJ, Douglas KM. Changes in Mood, Anxiety, and Cognition with Polycystic Ovary Syndrome Treatment: A Longitudinal, Naturalistic Study. Neuropsychiatr Dis Treat. 2022;18:2703-12. [View at publisher] [DOI] [PMID] [Google Scholar]
13. ESHRE TR, Group A-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and sterility. 2004;81(1):19-25. [View at publisher] [DOI] [PMID] [Google Scholar]
14. Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18(5):671-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Kim JJ. Obesity and Polycystic Ovary Syndrome. J Obes Metab Syndr 2024;33(4):289-301. [View at publisher] [DOI] [PMID] [Google Scholar]
16. Shele G, Genkil J, Speelman D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J Funct Morphol Kinesiol. 2020;5(2):35. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Yousuf SD, Ganie MA, Urwat U, Andrabi SM, Afzal Zargar M, Dar MA, et al. Oral contraceptive pill (OCP) treatment alters the gene expression of intercellular adhesion molecule-1 (ICAM-1), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) and plasminogen activator inhibitor-1 (PAI-1) in polycystic ovary syndrome (PCOS) women compared to drug-naive PCOS women. BMC Women's Health. 2023;23(1):68. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Escobar-Morreale HF, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18(2):146-70. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Long T, Zhang Y, Zeng C, Zheng S, Zhou L, Liu H. Effects of Low-Dose Spironolactone Combined with Metformin or Either Drug Alone on Insulin Resistance in Patients with Polycystic Ovary Syndrome: A Pilot Study. Int J Endocrinol. 2022;2022:9927240. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Attia GM, Almouteri MM, Alnakhli FT. Role of Metformin in Polycystic Ovary Syndrome (PCOS)-Related Infertility. Cureus. 2023;15(8):e44493. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Shah D, Patil M. National PCOS Working Group. Consensus Statement on the Use of Oral Contraceptive Pills in Polycystic Ovarian Syndrome Women in India. J Hum Reprod Sci. 2018;11(2):96-118. [View at Publisher] [DOI] [PMID] [Google Scholar]
22. Messinis IE. Ovulation induction: a mini review. Hum Reprod. 2005;20(10):2688-97. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Takasaki A, Tamura I, Okada-Hayashi M, Orita T, Tanabe M, Maruyama S, et al. Usefulness of intermittent clomiphene citrate treatment for women with polycystic ovarian syndrome that is resistant to standard clomiphene citrate treatment. Reprod Med Biol. 2018;17(4):454-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Kar S. Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial. J Hum Reprod Sci. 2012;5(3):262-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Sam S, Ehrmann DA. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia. 2017; 9(60):1656-61. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Nantia EA, Moundipa PF, Monsees TK, Carreau S. Medicinal plants as potential male anti-infertility agents: a review. Basic and Clinical Andrology. 2009;19(3):148-58. [View at Publisher] [DOI] [Google Scholar]
27. Khanage SG, Subhash TY, Bhaiyyasaheb IR. Herbal drugs for the treatment of Polycystic ovary syndrome (PCOS) and its complications. Pharmaceutical Research. 2019;2(1):5-13. [View at Publisher] [Google Scholar]
28. Asgarpanah J, Mohajerani R. Phytochemistry and pharmacologic properties of Urtica dioica L. Journal of medicinal plants research. 2012;6(46):5714-9. [View at Publisher] [DOI] [Google Scholar]
29. Mavi A, Terzi Z, Özgen U, Yildirim A, Coşkun M. Antioxidant properties of some medicinal plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), malva neglecta (malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), urtica dioica (urticaceae). Biol Pharm Bull. 2004;27(5):702-5. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Gülçin I, Küfrevioǧlu Öİ, Oktay M, Büyükokuroǧlu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004;90(2-3):205-15. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Koch E. Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms. Planta med. 2001;67(06):489-500. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Hadizadeh I, Peivastegan B, Kolahi M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pak J Biol Sci. 2009;12(1):58-63. [View at publisher] [DOI] [PMID] [Google Scholar]
33. Zare S, Nabiuni M, Tayanloo A, Hoseini S, Karimzadeh-Bardei L. The effects of Urtica dioica extract on lipid profile, insulin resistance index and liver histology in polycystic ovary syndrome-induced Wistar rats. Am J Chin Med. 2015;1(2):23-33. [View at Publisher] [Google Scholar]
34. Shakibaei M, Allaway D, Nebrich S, Mobasheri A. Botanical extracts from rosehip (Rosa canina), willow bark (Salix alba), and nettle leaf (Urtica dioica) suppress IL-1-induced NF-κB activation in canine articular chondrocytes. Evid Based Complement Alternat Med. 2012;2012. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Zemmouri H, Sekiou O, Ammar S, El Feki A, Bouaziz M, Messarah M, et al. Urtica dioica attenuates ovalbumin-induced inflammation and lipid peroxidation of lung tissues in rat asthma model. Pharm Biol. 2017;55(1):1561-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Azin F, Khazali H. Phytotherapy of polycystic ovary syndrome: A review. Int J Reprod Biomed. 2022;20(1):13-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
37. Momeni H, Salehi A, Seraji A. study of Vitex Agnus Castus and Evening Primrose oil on Body Mass Index (BMI). CMJA. 2012;2(2):194-203 [View at Publisher] [Google Scholar]
38. Attarzadeh HR, Sardar M, Taghavi M, Ayaz KHF . The effects of an aerobic exercise program on LH, FSH, TST and DHEA levels in obese women with polycystic ovary syndrome. IJEM. 2012;14(1).39-46. [View at Publisher] [Google Scholar]
39. Higham JM, O'brien P, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97(8):734-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Ramezani Tehrani F, Minooee S, Simbar M, Azizi F. A simpler diagnostic method to assess hirsutism in the Iranian population: Based on modified Ferriman-Gallwey scoring system (Tehran lipid and glucose study). Iranian Journal of Endocrinology and Metabolism. 2013;15(3):303-10. [View at Publisher] [Google Scholar]
41. Najafipour F, Rahimi AO, Mobaseri M, Agamohamadzadeh N, Nikoo A, Aliasgharzadeh A. Therapeutic effects of stinging nettle (Urtica dioica) in women with Hyperandrogenism. Int J Current Res Acad Rev. 2014;2(7):153-60 [View at Publisher] [Google Scholar]
42. Esfade H, Mirabolghasemi G, Azarnia M. The joint effect of hydro-alcoholic extract of nettle root and metformin on ovarian tissue of diabetic model of Wistar rat. Nova Biologica Reperta. 2019;6(2):131-9. [View at Publisher] [DOI] [Google Scholar]
43. Kianbakht S, Khalighi-Sigaroodi F, Dabaghian FH. Improved glycemic control in patients with advanced type 2 diabetes mellitus taking Urtica dioica leaf extract: a randomized double-blind placebo-controlled clinical trial. Clin Lab. 2013;59(9-10):1071-6. [View at Publisher] [DOI] [PMID] [Google Scholar]
44. Fakhraee S, Jouyandeh Z, Mehri A, Larijani B, Hasaniranjbar S. Systematic review on the effectiveness and safety of nettle herb in treating diabetes. J Diabetes Metab Disord. 2012;12(6):507-23. [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



