Volume 18, Issue 2 (7-2021)
J Res Dev Nurs Midw 2021, 18(2): 41-44 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Lashgari L, Jalal Manesh S, Kazem Naeini M. Effect of Maternal Empowerment Training on Frequency of Gastrointestinal Complications in Children Undergoing Chemotherapy. J Res Dev Nurs Midw 2021; 18 (2) :41-44
URL: http://nmj.goums.ac.ir/article-1-1072-en.html
URL: http://nmj.goums.ac.ir/article-1-1072-en.html
1- Faculty of Nursing and Midwifery, Azad University of Medical Sciences, Tehran, Iran , leilalashkari872@gimal.com
2- Department of Midwifery, Azad University of Medical Sciences, Tehran, Iran
3- Department of Statistics, Faculty of Nursing and Midwifery, Azad University of Medical Sciences, Tehran, Iran
2- Department of Midwifery, Azad University of Medical Sciences, Tehran, Iran
3- Department of Statistics, Faculty of Nursing and Midwifery, Azad University of Medical Sciences, Tehran, Iran
Full-Text [PDF 626 kb]
(1194 Downloads)
| Abstract (HTML) (3457 Views)

Full-Text: (741 Views)
Highlights:
What is current knowledge?
Knowledge about pediatric cancer treatments, including chemotherapy, is high, but little is known about parent education methods to reduce the effects of chemotherapy.
What is new here?
Different methods are used to educate the parents of sick children The empowerment model helps mothers to become more aware of the disease and its complications and to have more skills in managing the disease and its complications.
Introduction
Cancer is the second leading cause of death after cardiovascular disease in the United States (1). Childhood cancers are rare and account for only 1% of new cancer cases (2) but may be life threatening (3). There are various types of cancer in children that are different from those seen in adults. For instance, lymphoma, leukemia and brain tumors can be seen in more than half of children with cancer but account for less than 10% of cancer cases in adults (4). Childhood cancer is the second leading cause of death in children under 14 years of age in Iran (5). Fortunately, the advancement of science has increased the survival of cancer patients, and many treatment strategies have been discovered to cure these patients. Chemotherapy is commonly used for treatment of cancer, particularly childhood cancers. It refers to the use of drugs in order to eliminate cancer cells or slow down their growth. Chemotherapy aims to reduce or eliminate symptoms of cancers and is used alone or along with other treatments such as surgery or radiation therapy (6-8). Chemotherapy is more applicable in children than in adults because of the higher efficacy. In addition, children can better tolerate the side effects of chemotherapy compared to adults. Radiotherapy is less commonly used in children because of the long-term side effects. Treatment of childhood cancer is often through outpatient chemotherapy approach using programmable infusion pumps, oral chemotherapy regimen and early discharge from hospital with outside-home care and treatment monitoring (9). Antineoplastic drugs target cell cycle and therefore affect both healthy and cancer cells (10, 11). Given the high prevalence of chemotherapy complications in adults and children, it is essential to try limiting the side effects in order to prevent reduced quality of life in cancer patients, particularly children (12). In a study on chemotherapy complications in adolescents, 59% of adolescents reported that complications of anti-cancer therapies were worse than the cancer itself (13). Other studies on patients undergoing chemotherapy indicated some degrees of oral mucositis that lasted about 7 to 14 days (14, 15). In another study, the most common side effects of chemotherapy-related gastrointestinal complications included nausea, vomiting, aphthous stomatitis, diarrhea and odynophagia in children undergoing chemotherapy (13).
Children are dependent on their families for receiving care; hence, the presence of an active family member in child care is highly effective in the treatment outcome. In most families, mothers play the primary role in child care (16). In the past two decades, treatment of various diseases has shifted from hospitals to houses. More than 90% of cancer care and treatment are also carried out in an outpatient manner and at home. Teaching the patients, their family members and caregivers as well as active participation in providing care facilitate the care transfer from hospital to home (17). Therefore, empowerment is an important approach to engage and educate patients and caregivers (18). Empowerment, as a collaborative learning approach, requires close attention to the family and its needs as the center of care. Helping individuals and families to acquire an active role in health care is more important than aiding empowerment (17, 19). The Iranian empowerment model is derived from a research-based grounded theory based on Bandura's theory to improve chronic diseases. The model aims at empowering families to improve health and involves threat perception, problem-solving, educational participation and evaluation (20). This model also strengthens independence and respects the patient and family's choices, values, beliefs and cultural backgrounds (21). Given the importance of this model and the insufficient research on its impact on children with cancer, the present study aimed to examine effect of maternal empowerment training on frequency of gastrointestinal complications in children undergoing chemotherapy.
Methods
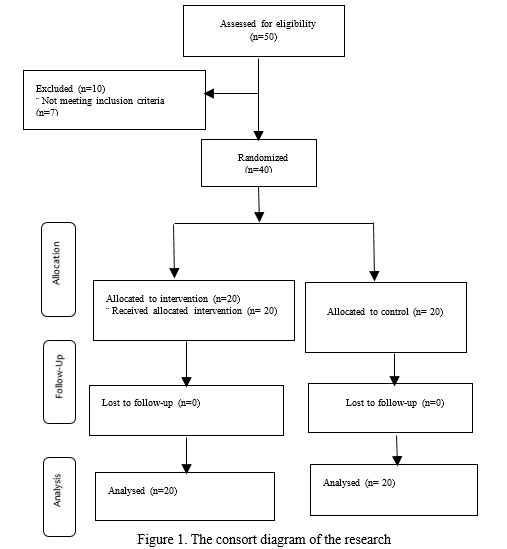
This clinical trial study was conducted in 2017 with a pretest-posttest design. The statistical population included 50 mothers of children with cancer undergoing chemotherapy at the Bahrami Children's Hospital in Tehran, Iran. The samples size was calculated based on the results of a pilot study and according to the sample size formula with a 95% confidence level and 90% test power. The minimum sample size was estimated to be 15 per group that was increased to 20 according to 30% drop out. Inclusion criterion was having a child less than 12 years of age with a definite diagnosis of cancer receiving chemotherapy (at least one or maximum two courses). Exclusion criteria included having debilitating diseases, receiving especial training courses such as chemotherapy training, working as healthcare personnel, unwillingness to continue cooperation and child's death. Overall, 40 eligible mothers were enrolled using the convenience sampling method. The subjects were then randomly assigned to an intervention (n=20) and a control (n=20) group (Fig. 1).
What is current knowledge?
Knowledge about pediatric cancer treatments, including chemotherapy, is high, but little is known about parent education methods to reduce the effects of chemotherapy.
What is new here?
Different methods are used to educate the parents of sick children The empowerment model helps mothers to become more aware of the disease and its complications and to have more skills in managing the disease and its complications.
Introduction
Cancer is the second leading cause of death after cardiovascular disease in the United States (1). Childhood cancers are rare and account for only 1% of new cancer cases (2) but may be life threatening (3). There are various types of cancer in children that are different from those seen in adults. For instance, lymphoma, leukemia and brain tumors can be seen in more than half of children with cancer but account for less than 10% of cancer cases in adults (4). Childhood cancer is the second leading cause of death in children under 14 years of age in Iran (5). Fortunately, the advancement of science has increased the survival of cancer patients, and many treatment strategies have been discovered to cure these patients. Chemotherapy is commonly used for treatment of cancer, particularly childhood cancers. It refers to the use of drugs in order to eliminate cancer cells or slow down their growth. Chemotherapy aims to reduce or eliminate symptoms of cancers and is used alone or along with other treatments such as surgery or radiation therapy (6-8). Chemotherapy is more applicable in children than in adults because of the higher efficacy. In addition, children can better tolerate the side effects of chemotherapy compared to adults. Radiotherapy is less commonly used in children because of the long-term side effects. Treatment of childhood cancer is often through outpatient chemotherapy approach using programmable infusion pumps, oral chemotherapy regimen and early discharge from hospital with outside-home care and treatment monitoring (9). Antineoplastic drugs target cell cycle and therefore affect both healthy and cancer cells (10, 11). Given the high prevalence of chemotherapy complications in adults and children, it is essential to try limiting the side effects in order to prevent reduced quality of life in cancer patients, particularly children (12). In a study on chemotherapy complications in adolescents, 59% of adolescents reported that complications of anti-cancer therapies were worse than the cancer itself (13). Other studies on patients undergoing chemotherapy indicated some degrees of oral mucositis that lasted about 7 to 14 days (14, 15). In another study, the most common side effects of chemotherapy-related gastrointestinal complications included nausea, vomiting, aphthous stomatitis, diarrhea and odynophagia in children undergoing chemotherapy (13).
Children are dependent on their families for receiving care; hence, the presence of an active family member in child care is highly effective in the treatment outcome. In most families, mothers play the primary role in child care (16). In the past two decades, treatment of various diseases has shifted from hospitals to houses. More than 90% of cancer care and treatment are also carried out in an outpatient manner and at home. Teaching the patients, their family members and caregivers as well as active participation in providing care facilitate the care transfer from hospital to home (17). Therefore, empowerment is an important approach to engage and educate patients and caregivers (18). Empowerment, as a collaborative learning approach, requires close attention to the family and its needs as the center of care. Helping individuals and families to acquire an active role in health care is more important than aiding empowerment (17, 19). The Iranian empowerment model is derived from a research-based grounded theory based on Bandura's theory to improve chronic diseases. The model aims at empowering families to improve health and involves threat perception, problem-solving, educational participation and evaluation (20). This model also strengthens independence and respects the patient and family's choices, values, beliefs and cultural backgrounds (21). Given the importance of this model and the insufficient research on its impact on children with cancer, the present study aimed to examine effect of maternal empowerment training on frequency of gastrointestinal complications in children undergoing chemotherapy.
Methods
This clinical trial study was conducted in 2017 with a pretest-posttest design. The statistical population included 50 mothers of children with cancer undergoing chemotherapy at the Bahrami Children's Hospital in Tehran, Iran. The samples size was calculated based on the results of a pilot study and according to the sample size formula with a 95% confidence level and 90% test power. The minimum sample size was estimated to be 15 per group that was increased to 20 according to 30% drop out. Inclusion criterion was having a child less than 12 years of age with a definite diagnosis of cancer receiving chemotherapy (at least one or maximum two courses). Exclusion criteria included having debilitating diseases, receiving especial training courses such as chemotherapy training, working as healthcare personnel, unwillingness to continue cooperation and child's death. Overall, 40 eligible mothers were enrolled using the convenience sampling method. The subjects were then randomly assigned to an intervention (n=20) and a control (n=20) group (Fig. 1).

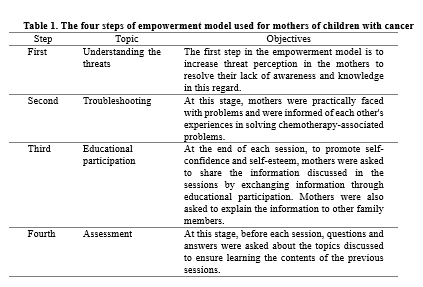
The study received approval from the ethics committee of Islamic Azad University, Tehran Medical Sciences Branch (ethics code: IR.IAU.TMU.REC.1396.64). Written informed consent was taken from all participants after detailed explanation of the study objectives. Data collection tools included a demographic information (age, sex, marital status, education level, etc.) questionnaire and a checklist of symptoms of acute and common gastrointestinal complications of chemotherapy in children. The demographic information questionnaire was completed by the researcher. The checklist included 14 questions on symptoms of gastrointestinal complications that were selected according to oncology and pediatric textbooks (1, 9, 22- 24) and the side effect checklist of the American Cancer Society (25). The content validity indicator and content validity ratio were used to confirm the content validity of the checklist. The following criteria were used in order to calculate the content validity indicator: "simplicity and fluency", "clarity or transparency" and "relevance". Based on the results, all items were accepted with a content validity indicator of greater than 83%. The checklist’s reliability was also approved by obtaining a Cronbach's alpha coefficient of 0.88. The empowerment training was only held for the intervention group and the control group received no training. The training consisted of five one-hour sessions held in groups of five individuals (Table 1). The researcher completed the checklist for both groups at baseline and two, four and eight weeks after the last training session. At the end of the study, all issues relating to empowerment and training sessions were given to the control group in forms of CDs and booklet. Data were analyzed in SPSS Statistics for Windows, version 16 (SPSS Inc., Chicago, Ill., USA). All statistical analyses were carried out at significance of 0.05.

Results
The mean age of children and mothers was 4.75±2.25 and 31.33±4.40 years, respectively. All children had health insurance. The frequency of girls in the intervention and the control groups was 75% and 35%, respectively. Most of the subjects in the intervention group (70%) and the control group (90%) were not students. All mothers were married. In addition, 55% of mothers in the intervention group had high school diploma and 45% of mothers in the control group had secondary education. Moreover, 85% of subjects in the intervention group and 90% of subjects in the control group were mothers' assistants in child care.
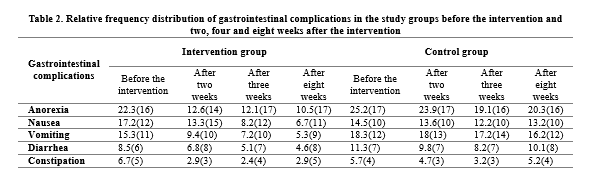
As shown in table 2, there was no significant difference between the two groups in terms of the frequency of gastrointestinal complications before the intervention. However, the frequency of gastrointestinal complications was lower in the intervention group than in the control group two, four and eight weeks after the empowerment training.
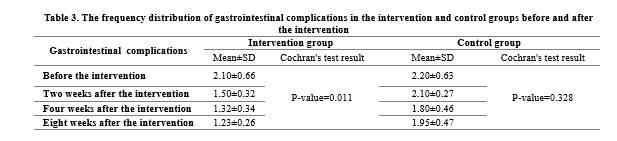
The results of the chi-square test indicated no significant difference between the two groups in terms of gastrointestinal complications before the intervention, but the Cochran's test results confirmed a significant reduction in the frequency of gastrointestinal complications in the intervention group after the empowerment training (P<0.05). The results of the McNemar test in binary comparisons indicated that the frequency of the complications decreased significantly two weeks after the intervention compared to baseline (P=0.033). This significant decrease was observed in the following evaluations (P=0.037 in the fourth week and P=0.038 in the eighth week). The chi-square test results also indicated that gastrointestinal complications were significantly lower in the intervention group than in the control group in all posttest (P<0.05) (Table 3).
In the control group, the frequency of gastrointestinal complications was highest (75%) in the pretest and lowest (60%) four weeks after chemotherapy (P>0.05). Results of the chi-square test indicated that the frequency of gastrointestinal complications was significantly lower in the intervention group than in the control group in all posttest stages (P<0.05) (Table 4).
Discussion
According to the results, teaching mothers decreased the incidence and severity of gastrointestinal complications of chemotherapy in children with cancer. This is consistent with the results of a study by Asgarabad et al. (26). Similarly, Karimi et al. (2017) also showed that an educational program could significantly reduce the frequency of nausea and vomiting in cancer patients undergoing chemotherapy. They concluded that the training program could be considered a complementary approach for anti-inflammatory drugs (27).
In a study by Partovi et al. (2013), the prevalence of gastrointestinal complications was high in children with leukemia undergoing chemotherapy (13). In another study by Sizen et al. (2012), the prevalence of gastrointestinal complications and acute constipation in cancer children undergoing chemotherapy was high (24). In a study by Cheng (2008), self-care and quality of life increased and the frequency of chemotherapy side effects decreased after a training program and telephone follow-up for four weeks (25). In line with our findings, Karimi et al. (2014) reported that a training program could significantly reduce chemotherapy-related gastrointestinal complications in patients (26). The implementation of empowerment models is more effective than regular trainings in reducing the frequency of chemotherapy-associated side effects. In other words, modern methods of training along with commonly used methods, such as learning through booklets, telephone follow-up and continuous training could improve mothers and patients' knowledge about chronic diseases. In this regard, Davarpanah et al. demonstrated that the empowerment model was effective in improving the patient training (27).
We did not consider allergy to medications, patients’ immunity level and diet during the study period, which is a limitation of this study. Therefore, it is recommended to consider these possible confounding factors in future studies.
Conclusion
The empowerment training increased knowledge and understanding of the mothers about symptoms and reduced the frequency of gastrointestinal complications following chemotherapy. Therefore, implementation of such training programs could help increase quality of life and the treatment outcome in children undergoing chemotherapy.
Acknowledgements
We will like to extend our gratitude to all participants for their cooperation in this research.
Funding source
There are no conflicts of interest to declare.
Ethical statement
The present manuscript was derived from a master's thesis approved by the Islamic Azad University, Tehran Medical Sciences, Iran. This clinical trial was also registered on the Iranian Registry of Clinical Trials (IRCT20180121038467N1). We are grateful to the staff of the Islamic Azad University of Tehran and Bahrami Children's Hospital as well as patients and their families for their cooperation.
Conflict of interest
The authors declare that there is no conflict of interest
The mean age of children and mothers was 4.75±2.25 and 31.33±4.40 years, respectively. All children had health insurance. The frequency of girls in the intervention and the control groups was 75% and 35%, respectively. Most of the subjects in the intervention group (70%) and the control group (90%) were not students. All mothers were married. In addition, 55% of mothers in the intervention group had high school diploma and 45% of mothers in the control group had secondary education. Moreover, 85% of subjects in the intervention group and 90% of subjects in the control group were mothers' assistants in child care.
As shown in table 2, there was no significant difference between the two groups in terms of the frequency of gastrointestinal complications before the intervention. However, the frequency of gastrointestinal complications was lower in the intervention group than in the control group two, four and eight weeks after the empowerment training.
The results of the chi-square test indicated no significant difference between the two groups in terms of gastrointestinal complications before the intervention, but the Cochran's test results confirmed a significant reduction in the frequency of gastrointestinal complications in the intervention group after the empowerment training (P<0.05). The results of the McNemar test in binary comparisons indicated that the frequency of the complications decreased significantly two weeks after the intervention compared to baseline (P=0.033). This significant decrease was observed in the following evaluations (P=0.037 in the fourth week and P=0.038 in the eighth week). The chi-square test results also indicated that gastrointestinal complications were significantly lower in the intervention group than in the control group in all posttest (P<0.05) (Table 3).
In the control group, the frequency of gastrointestinal complications was highest (75%) in the pretest and lowest (60%) four weeks after chemotherapy (P>0.05). Results of the chi-square test indicated that the frequency of gastrointestinal complications was significantly lower in the intervention group than in the control group in all posttest stages (P<0.05) (Table 4).
Discussion
According to the results, teaching mothers decreased the incidence and severity of gastrointestinal complications of chemotherapy in children with cancer. This is consistent with the results of a study by Asgarabad et al. (26). Similarly, Karimi et al. (2017) also showed that an educational program could significantly reduce the frequency of nausea and vomiting in cancer patients undergoing chemotherapy. They concluded that the training program could be considered a complementary approach for anti-inflammatory drugs (27).
In a study by Partovi et al. (2013), the prevalence of gastrointestinal complications was high in children with leukemia undergoing chemotherapy (13). In another study by Sizen et al. (2012), the prevalence of gastrointestinal complications and acute constipation in cancer children undergoing chemotherapy was high (24). In a study by Cheng (2008), self-care and quality of life increased and the frequency of chemotherapy side effects decreased after a training program and telephone follow-up for four weeks (25). In line with our findings, Karimi et al. (2014) reported that a training program could significantly reduce chemotherapy-related gastrointestinal complications in patients (26). The implementation of empowerment models is more effective than regular trainings in reducing the frequency of chemotherapy-associated side effects. In other words, modern methods of training along with commonly used methods, such as learning through booklets, telephone follow-up and continuous training could improve mothers and patients' knowledge about chronic diseases. In this regard, Davarpanah et al. demonstrated that the empowerment model was effective in improving the patient training (27).
We did not consider allergy to medications, patients’ immunity level and diet during the study period, which is a limitation of this study. Therefore, it is recommended to consider these possible confounding factors in future studies.
Conclusion
The empowerment training increased knowledge and understanding of the mothers about symptoms and reduced the frequency of gastrointestinal complications following chemotherapy. Therefore, implementation of such training programs could help increase quality of life and the treatment outcome in children undergoing chemotherapy.
Acknowledgements
We will like to extend our gratitude to all participants for their cooperation in this research.
Funding source
There are no conflicts of interest to declare.
Ethical statement
The present manuscript was derived from a master's thesis approved by the Islamic Azad University, Tehran Medical Sciences, Iran. This clinical trial was also registered on the Iranian Registry of Clinical Trials (IRCT20180121038467N1). We are grateful to the staff of the Islamic Azad University of Tehran and Bahrami Children's Hospital as well as patients and their families for their cooperation.
Conflict of interest
The authors declare that there is no conflict of interest
Author contributions
All authors have contributed significantly to this study and the preparation of this manuscript and we agreed with its contents. The specific contributions include: Leila Lashgari , Shamsolmoluk Jalal Manesh in writing the background, reviewing the literature, and discussing the findings, while Leila Lashgari ,Mohammad Kazem Naeini did the data collection and analysis



Type of study: Original Article |
Subject:
Nursing
References
1. Hinkel El J. Brunner-Sudhart Internal Surgery Nursing. Translation by Neda Sanaei, Fereshteh Javaheri Tehrani. Tehran: Raffaq Publishing; 2014.
2. Karen, c. Nelson's Pediatric Medicine Basics. Translation by Farshad Moghadam, Pourranandakht Gholamipour Shirazi. Tehran: Artin Teb Publishing; 2015.
3. Heron M. Deaths: leading causes for 2008. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012; 60(6):1-94. [View at paplisher] [Google Scholar]
4. Downing J, Wilson R.K, Zhang J, Mardis E.R، Pui C.H, Ding L, et all. The pediatric cancer genome project. Nature genetics. 2012 Jun; 44 (6):619-22 [View at paplisher] [DOI] [PMID] [Google Scholar]
5. Sloper P. Predictors of distress in parents of children with cancer: A prospective study. Journal of pediatric psychology. 2000 Mar 1; 25(2):79-91. [View at paplisher] [DOI] [PMID] [Google Scholar]
6. Bruce J, Pelosi E. Understanding Chemotherapy, A guide for people with cancer, their families and friends. Cancer Council Australia. 2016; ISBN 9781925136180.
7. Moafi A, Soheilipor F, Amini A, Beheshti M. Comparing efficacy and side effects of Pd-Grastim and Neupogen for prevention of neutropenia after chemotherapy in children. Disease Childhood Iran 2005; 2 (16):143-8. [Persian] [View at paplisher] [Google Scholar]
8. Momtazmanesh N, Vaziri S, Taghadosi M. characteristics of children with leukemia during the Kashan region. J Feyz, 2000; 1(14):103-9. [Persian] [View at paplisher]
9. Cligman, R. Nelson Oncology Diseases. Saba Maher, translator,. Tehran: Andisheh Rafi Publishing; 2016.
10. Rajagopala SV, Vashee S, Oldfield LM, Suzuki Y, Venter JC, Telenti A, Nelson KE. The human microbiome and cancer. Cancer Prevention Research. 2017;10(4):226-34. [View at paplisher] [DOI] [PMID] [Google Scholar]
11. Pearce A, Haas M, Viney R, Pearson SA, Haywood P, Brown C, Ward R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PloS one. 2017 Oct 10;12(10):e0184360. [View at paplisher] [DOI] [PMID] [Google Scholar]
12. Farhat A. feeding in pediatric diseases. Mashhad: Mashhad University of Medical Sciences; 2008 [Persian]
13. Partovi S, Banihashem A, Farshidi F. Prevalence of gastrointestinal side effects of chemotherapy in children with leukemia. Iran J Pediat. 2013; 2(14):89-124. [Persian]
14. Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, et all. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2004; 100(S9):2026-46. [View at paplisher] [DOI] [PMID] [Google Scholar]
15. Sonis ST. The pathobiology of mucositis. Nature Reviews Cancer. 2004; 4(4):277-84. [View at paplisher] [DOI] [PMID] [Google Scholar]
16. Miller M, Kearney N. Chemotherapy‐related nausea and vomiting-past reflections, present practice and future management. European Journal of cancer care. 2004; 13(1):71-81. [View at paplisher] [DOI] [PMID] [Google Scholar]
17. Azmoude, E., Jaafarnejad, F., Mazloum, S. Effect of self-efficacy-based training on maternal sense of competency of primiparous women in the infant's care. Evidence Based Care, 2014; 4(3): 7-14. [Persian] [View at paplisher] [Google Scholar]
18. Farahani Behnaz, Safavi Mahboubeh, Salehi Shiva. Evaluating the effect of instructing patient care on knowledge, attitude and performance of the attentives of cancer patients under chemotherapy referring to the university hospitals of Tehran University of Medical Sciences. MEDICAL SCIENCES. 2004; 14 (2) :99-103. [Persian] [View at paplisher] [Google Scholar]
19. Dalvand H, Rassafiani M, Bagheri H. Family Centered Approach: A literature the review. mrj. 2014; 8 (1):1-9. [Persian] [View at paplisher] [Google Scholar]
20. Khosravan Sh, Kolbadinezhad N, Alami A, Torabi Sh. Effect of intervention based on family- centered empowerment model on the quality of life of women suffering from stress urinary incontinence. 2014; 19(5): 271-280. [Persian] [Google Scholar]
21. Masoodi R, Soleimani M A, Alhani F, Rabiei L, Bahrami N, Esmaeili S A. Effects of family-centered empowerment model on perceived satisfaction and self-concept of multiple sclerosis patients care givers. Koomesh. 2013; 14 (2) :240-248. [Persian] [View at paplisher] [Google Scholar]
22. Elahi Asgarabad, H., Behnam Vashani, H., Badiei, Z., Heshmati Nabavi, F., Malekzadeh, J. Effect of Empowering Caregivers of Children with Cancer Undergoing Chemotherapy on Their Adherence to Preventive Health Recommendations for Oral Ulcer. Evidence Based Care, 2014; 4(3): 23-32. [Persian] [View at paplisher] [Google Scholar]
23. Karimi S, Makhsosi BR, Seyedi-Andi SJ, Behzadi M, Moghofeh Y, Mohammadinasrabadi K, Abdi A, Ahmadi P. Surveying the effect of a self-care education program on severity of nausea and emesis in colorectal cancer patients under chemotherapy. Journal of multidisciplinary healthcare. 2017; 10:301. [View at paplisher] [DOI] [PMID] [Google Scholar]
24. Season J. Acute Constipation in Children Receiving Chemotherapy for Cancer (2012). Yale Medicine Thesis Digital Library. 2012. [View at paplisher] [Google Scholar]
25. Chung Y-C, Hwang H. Education for homecare patients with leukemia following a cycle of chemotherapy: an exploratory pilot study.Oncology nursing forum; 2008: onc nurs society. [View at paplisher] [DOI] [Google Scholar]
26. Karimi S, Vanaki Z, Bashiri H, Hassani S.A. The effect of Orem self-care ability of patients with colorectal cancer. Avicenna J Nurs Midwifery Care. 2016; 24 (2):105-112. (Persian) [View at paplisher] [Google Scholar]
27. Davarpanah M, Fayazi S, Shariati A Davoud Mirhosseini S. The Effect of Family-Centered Empowerment Model on the Quality of Life of Patients with Leukemia. Jundishapur J Chronic Dis Care. 2017 January; 6(1): 36441. [Persian] [View at paplisher] [DOI] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






